The impact of aortic valve replacement (AVR) on the dynamic geometry and motion of the mitral annulus remains unknown. We analyzed the effects of AVR on the dynamic geometry and motion of the mitral annulus. We used 3-dimensional transesophageal echocardiography to analyze 39 consecutive patients undergoing elective surgical AVR for aortic stenosis. Intraoperative 3-dimensional transesophageal echocardiography was performed immediately before and after AVR. Volumetric data sets were analyzed using a software package capable of dynamically tracking the mitral annulus and leaflets during the entire systolic ejection phase. After AVR, there were significant decreases (p <0.01) in annular dimensions such as anteroposterior (3.5 ± 0.1 vs 3.2 ± 0.1 cm), anterolateral-posteromedial (3.7 ± 0.1 vs 3.5 ± 0.1 cm), and commissural diameters (3.7 ± 0.1 vs 3.3 ± 0.1 cm), as well as annular circumference (12.0 ± 0.30 vs 11.1 ± 0.2 cm) and 3-dimensional mitral annular area (mean 10.9 ± 0.6 vs 9.3 ± 0.3 cm 3 ). Vertical mitral annular displacement was also reduced (6.2 ± 3.1 vs 4.3 ± 2.2 mm). Mitral annular nonplanarity angle (154 ± 1.5° vs 161 ± 1.6°) and aorto-mitral angle (133 ± 3.3° vs 142 ± 2.0°) were both increased after AVR, suggesting reduced nonplanar shape of the mitral annulus and reduced aorto-mitral flexion. In conclusion, these data demonstrate that mitral annular size is reduced immediately after AVR and that the dynamic motion of the mitral annulus is restricted. These findings may have important clinical implications for patients undergoing AVR with concurrent mitral regurgitation.
Aortic valve replacement (AVR) for aortic stenosis is the most common valve procedure performed in the United States. Observational data have noted that most patients undergoing AVR have at least mild mitral regurgitation (MR). Of these, 40% to 80% of patients with functional MR and 27% to 44% of patients with organic MR experience significant reduction in the severity of MR after AVR. This reduction has been attributed to a decrease in afterload and subsequent ventricular remodeling. The anatomic proximity between the aortic and mitral valves (AV and MV) makes it intuitive that aorto-mitral coupling could be mechanically affected by a prosthesis in the aortic position. Three-dimensional (3D) transesophageal echocardiography (TEE) has recently demonstrated mitral annular area reduction after AVR that could partly be because of mechanical compression by the prosthesis. However, it remains unknown whether these geometric mitral annular changes are sustained throughout the cardiac cycle. In this study, we sought to ascertain whether changes in the mitral annulus after AVR are maintained throughout the systolic phase of the cardiac cycle and whether there were any additional dynamic changes in mitral annular geometry.
Methods
Consecutive patients presenting to our medical center from June 2009 to August 2011 for elective surgical AVR for aortic stenosis were enrolled. All patients underwent intraoperative 3D transesophageal echocardiographic examinations as part of routine clinical care. Patients who had undergone previous MV repair, had a prosthetic device in the mitral position, and/or underwent concurrent MV replacement, or had a contraindication to TEE were excluded. This study was approved by our Institutional Review Board with waiver of informed consent.
The pre-AVR transesophageal echocardiographic examination was performed after induction of general anesthesia and before institution of cardiopulmonary bypass during a phase of hemodynamic stability. The post-AVR examination was performed after an aortic prosthesis had been successfully placed and successful separation from cardiopulmonary bypass. Echocardiographic imaging was performed with a Phillips Medical Systems iE33 ultrasound system (Andover, Massachusetts) equipped with the transesophageal X7-2t 3D matrix probe. The 2-dimensional (2D) examination was performed according to standardized guidelines, and MR was assessed using both visual grading and by way of the vena contracta method. This was followed by 3D image acquisition, and an en face view of the MV was acquired initially. The 3D image acquisition of the MV was initiated with the transesophageal echocardiographic probe in the mid-esophageal position and with R-wave gated acquisition over 3 to 4 heart beats during a brief period of induced apnea. These data sets were exported to an off-line Windows-based workstation for further analysis. Hemodynamic data were collected from the patients’ anesthesia records, and preoperative echocardiographic data were collected from the electronic medical record. Live zoom imaging was performed in patients in whom R-wave gating was not possible.
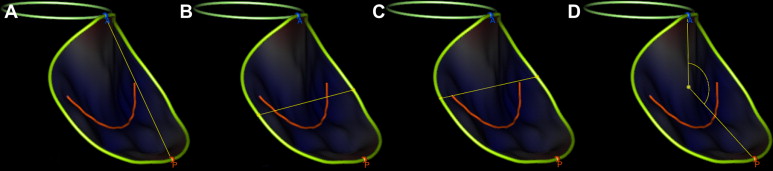
Off-line dynamic MV geometrical analyses were performed using TomTec 4D Mitral Valve Assessment 2.0 (TomTec Imaging Systems GmBH, Munich, Germany). The feasibility of this software for assessment of MV dynamic analysis has been previously reported and it has been shown to be both accurate and reproducible. This dynamic analysis occurs in a 7-step sequential workflow. The temporal boundaries of the dynamic analysis consist of end-diastole (last frame before the MV starts to close) to end-systole (last frame before the MV opens). This is followed by identification of mitral annular landmarks (anterior, posterior, medial, and lateral), the AV, and the coaptation point of the MV leaflets. The rest of the geometric analysis is automated with the generation of a static and a dynamic 3D model of the MV ( Figure 1 ). Analysis of MV geometry was blinded to pre- and post-AVR state. A smaller subset of patients was selected for intra- and interobserver variability analysis.
After the institution of cardiopulmonary bypass and aortic clamping, the AV was exposed after aortotomy. The valve cusps were excised, the root irrigated, and interrupted pledgeted Ethibond sutures (Ethicon Inc., Somerville, New Jersey) were placed in the aortic annulus with the pledgets on the ventricular side. In the case of calcific disease, the AV was also debrided. These sutures were then tied through the sewing portion of the prosthetic aortic ring that was placed in a suprannular position. The choice of aortic prosthesis was based on surgeon preference and patient indication. After the placement of the ring, aortotomy was closed, cross-clamp was released, and the patient was taken off cardiopulmonary bypass.
Data acquired from MV dynamic analysis can then be exported in “.csv (comma separated values)” file format that can be accessed using Microsoft Excel (Microsoft Corp., Richmond, Washington), which was used for data entry and organization. The number of frames and corresponding data points are dependent on the mode of 3D imaging, the patient’s heart rate, depth of imaging, and the temporal resolution of the imaging mode (which can be increased by increasing the number of cardiac cycles during which acquisition occurs). Variables were analyzed using JMP 8.0 software (SAS Institute Inc., Cary, North Carolina) with dependent paired t tests. p Values <0.05 were considered significant. Continuous variables are reported as mean values ± SDs.
Results
A total of 39 patients (74.0 ± 13.7 years) were enrolled, of whom 24 were men. Concurrent coronary artery bypass grafting was performed in 13 of them. Preoperative transthoracic echocardiographic parameters are listed in Table 1 . The average prosthetic ring size was 23 mm (range = 19 to 29 mm). Tissue valves were placed in 87% (n = 34) of patients and included 9 Medtronic Mosaic Ultra valves (Medtronic, Minneapolis, Minnesota), 17 St. Jude Biocor Epic valves (St. Jude Medical Inc., St. Paul, Minnesota), and 8 Carpentier-Edwards Magna Ease valves (Edwards Lifesciences Inc., Irvine, California). Among the mechanical valves were 4 St. Jude Medical Regent valves and 1 On-X valve (On-X Life Technologies Inc., Austin, Texas). Differences in intraoperative hemodynamic and echocardiographic parameters before and after AVR are listed in Table 2 . Although a significant reduction was noted in MR grade (p = 0.002), no significant change in the vena contracta was noted (0.07). Only 13 patients had more than mild MR before AVR. Three-dimensional image acquisition was successful in all patients with full volume data sets acquired in 34 patients; 5 patients underwent live zoom imaging as they had atrial fibrillation. Temporal resolution ranged from 3 to 17 frames (mean = 8 frames). Intra- and interobserver correlation was 0.93 and 0.84, respectively (both p <0.01).
| Parameters | Mean Preoperative Values | Normal Range |
|---|---|---|
| Left atrium long-axis length (cm) | 4.2 ± 0.9 | <4.0 |
| Left atrium 4-chamber length (cm) | 5.7 ± 0.9 | <5.2 |
| Left ventricle septal wall thickness (cm) | 1.3 ± 0.3 | 0.6–1.1 |
| Left ventricle inferolateral wall thickness (cm) | 1.3 ± 0.2 | 0.6–1.1 |
| Left ventricle diastolic dimension (cm) | 4.5 ± 0.6 | <5.2 |
| Transmitral E wave/Lateral mitral annulus E′ wave | 14.5 ± 5.5 | <15 |
| Aortic valve peak velocity (m/s) | 4.2 ± 0.8 | <2 |
| Aortic valve peak gradient (mm Hg) | 72.4 ± 27.5 | <20 |
| Aortic valve mean gradient (mm Hg) | 43.6 ± 15.4 | |
| Aortic valve area (cm 2 ) | 0.8 ± 0.3 | <3.0 |
| Parameters | Before AVR | After AVR | p Value |
|---|---|---|---|
| MR grade | 1.06 (0.87) | 0.48 (0.68) | 0.002 |
| Vena contracta (mm) | 0.46 (0.25) | 0.34 (0.21) | 0.07 |
| Coaptation 0 (degrees) | 0.77 (0.28) | 0.75 (0.33) | 0.8 |
| Coaptation 135 (degrees) | 0.76 (0.3) | 0.71 (0.28) | 0.5 |
| Annular diameter 0 (degrees) | 3.72 (0.86) | 3.34 (0.79) | 0.09 |
| Annular diameter 135 (degrees) | 3.34 (0.82) | 2.94 (0.69) | 0.06 |
| Left ventricular end-diastolic diameter (cm) | 4.78 (1.1) | 4.54 (0.69) | 0.6 |
| Central venous pressure (mm Hg) | 14.23 (2.86) | 13.51 (3.14) | 0.3 |
| Mean arterial pressure (mm Hg) | 80.64 (8.0) | 71.13 (6.25) | <0.0001 |
Dynamic changes in MV geometry are listed in Table 3 . A significant reduction was noted in all MV dimensions including anteroposterior, anterolateral-posteromedial, and commissural diameters, 2D annular area, 3D annular area, annular circumference, posterior leaflet area, and anterior leaflet area. Significant changes in parameters of mitral annular nonplanarity such as the nonplanarity angle, tenting height, and tenting volume indicated a reduction in mitral nonplanarity. The aorto-mitral angle was also increased after AVR. Most parameters also underwent greater change dynamically during the cardiac cycle before AVR compared with that after AVR ( Table 3 and Figure 2 ). Annular fraction, which is the percentage change that occurs in the annular area during systole, was also reduced (10.4% [8.0] vs 7.0% [4.0], p = 0.02). No change was demonstrated in the tenting volume fraction (26.0% [16.1] vs 27.0% [13.8], p = 0.7). A reduction in maximum vertical annular displacement (p <0.01; Figure 3 ) was noted without any change in vertical annular velocity (p = 0.4).
| Dynamic Change | 1—Start of Systole | 2—Early Systole | 3—Mid Systole | 4—Late Systole | 5—End of Systole | Mean Change | Maximal Delta Change (%) |
|---|---|---|---|---|---|---|---|
| Anteroposterior diameter (cm) | |||||||
| Before AVR | 3.3 (0.6) | 3.4 (0.8) | 3.4 (0.5) | 3.5 (0.5) | 3.5 (0.6) | 3.4 (0.1) | 6 |
| After AVR | 3.1 (0.7) | 3.2 (0.6) | 3.2 (0.6)*** | 3.2 (0.6)*** | 3.2 (0.6)** | 3.2 (0.04)* | 3 |
| Anterolateral-posteromedial diameter (cm) | |||||||
| Before AVR | 3.6 (0.6) | 3.7 (0.5) | 3.7 (0.5) | 3.8 (0.5) | 3.8 (0.6) | 3.7 (0.1) | 6 |
| After AVR | 3.4 (0.5) | 3.5 (0.5) | 3.4 (0.5)*** | 3.5 (0.5)*** | 3.5 (0.6)** | 3.5 (0.1)* | 3 |
| Commissural diameter (cm) | |||||||
| Before AVR | 3.5 (0.6) | 3.6 (0.5) | 3.7 (0.7) | 3.7 (0.5) | 3.7 (0.6) | 3.6 (0.1) | 6 |
| After AVR | 3.2 (0.6)*** | 3.4 (0.6) | 3.3 (0.5)** | 3.5 (0.9) | 3.3 (0.6)** | 3.3 (0.1)** | 9 |
| Circumference (cm) | |||||||
| Before AVR | 11.4 (1.9) | 11.6 (1.7) | 11.8 (1.6) | 12.0 (1.4) | 12.0 (1.9) | 11.8 (0.3) | 5 |
| After AVR | 10.7 (1.7) | 10.9 (1.5) | 10.8 (1.7)*** | 11.0 (1.6)** | 10.9 (1.8)** | 10.9 (0.1)* | 3 |
| Annular area (2D; cm 2 ) | |||||||
| Before AVR | 9.8 (3.3) | 10.0 (2.8) | 10.4 (2.7) | 10.8 (2.6) | 10.7 (3.3) | 10.3 (0.4) | 10 |
| After AVR | 8.6 (2.9) | 8.9 (2.6) | 8.9 (2.8)*** | 9.0 (2.7)*** | 8.8 (2.9)** | 8.8 (0.2)* | 5 |
| Annular area (3D; cm 2 ) | |||||||
| Before AVR | 10.0 (3.3) | 10.2 (2.8) | 10.6 (2.8) | 11.0 (2.6) | 11.0 (3.4) | 10.6 (0.5) | 10 |
| After AVR | 8.8 (3.0) | 9.0 (2.7) | 9.0 (2.8)*** | 9.2 (2.7)** | 9.0 (3.0)** | 9.0 (0.1)* | 5 |
| Anterior leaflet area (cm 2 ) | |||||||
| Before AVR | 6.6 (2.3) | 6.6 (1.9) | 6.6 (1.9) | 6.8 (1.8) | 6.5 (2.2) | 6.6 (0.1) | 5 |
| After AVR | 6.1 (2.3) | 6.2 (2.1) | 6.0 (2.2) | 6.2 (2.2) | 5.8 (2.2) | 6.1 (0.2)* | 7 |
| Posterior leaflet area (cm 2 ) | |||||||
| Before AVR | 6.1 (2.3) | 5.9 (2.1) | 6.0 (2.0) | 6.1 (1.9) | 6.2 (2.2) | 6.1 (0.1) | 5 |
| After AVR | 5.3 (2.2) | 5.2 (1.8) | 5.1 (1.9)*** | 5.2 (1.9) | 5.1 (2.0)*** | 5.2 (0.1)* | 4 |
| Nonplanarity angle (degrees) | |||||||
| Before AVR | 156.3 (16.0) | 153.3 (17.9) | 155.2 (16.1) | 154.1 (16.3) | 154.9 (15.5) | 154.8 (1.1) | 2 |
| After AVR | 160.8 (15.1) | 161.0 (16.7) | 160.7 (15.3) | 162.8 (16.9) | 158.7 (16.3) | 160.8 (1.5)** | 3 |
| Tenting volume (mm 3 ) | |||||||
| Before AVR | 3.4 (1.8) | 3.0 (1.0) | 2.9 (1.6) | 2.6 (1.2) | 2.6 (1.4) | 2.9 (0.3) | 23 |
| After AVR | 2.3 (1.3)** | 2.2 (1.0)*** | 2.0 (1.0)** | 1.9 (0.9)*** | 1.9 (1.0)*** | 2.1 (0.2)* | 22 |
| Tenting height (mm) | |||||||
| Before AVR | 8.3 (3.1) | 8.1 (2.8) | 7.3 (2.7) | 7.0 (2.7) | 7.1 (2.7) | 7.6 (0.6) | 19 |
| After AVR | 7.3 (2.9) | 7.0 (2.3) | 6.7 (2.7) | 6.3 (2.1) | 6.8 (2.7) | 6.8 (0.4)*** | 16 |
| Vertical annular displacement (mm) | |||||||
| Before AVR | 0 | 1.8 (1.0) | 3.8 (1.6) | 5.7 (2.2) | 6.0 (2.2) | ||
| After AVR | 0 | 1.7 (0.6) | 2.8 (1.2)** | 3.9 (1.5)* | 3.9 (1.5)* | ||
| Annular velocity (mm/s) | |||||||
| Before AVR | 13.1 (9.2) | 20.3 (8.4) | 22.6 (9.9) | 16.0 (8.4) | 8.9 (7.0) | 16.2 (5.5) | 154 |
| After AVR | 14.5 (8.2) | 18.5 (7.3) | 16.2 (6.5)** | 12.6 (8.0) | 11.1 (8.1) | 14.6 (2.9) | 67 |
| Aorto-mitral angle (degrees) | |||||||
| Before AVR | 133.1 (19.4) | 130.6 (19.0) | 134.2 (18.7) | 134.3 (20.2) | 139.4 (19.4) | 134.3 (3.2) | 7 |
| After AVR | 139.3 (17.0) | 140.2 (15.2) 0.04 | 140.4 (16.5) | 142.2 (16.9) | 143.7 (17.3) | 141.2 (1.8)** | 3 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


