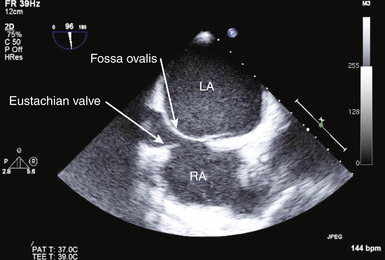
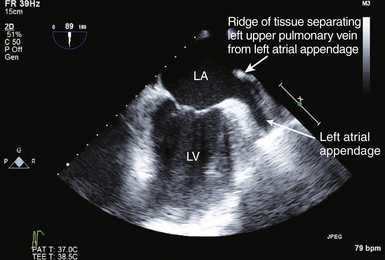
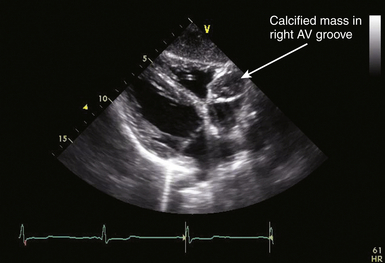
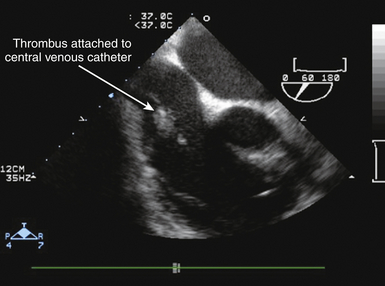
21 Many normal variants can be misinterpreted as pathologic abnormalities and are thus “pseudo-masses.” A number of intracardiac structures can be difficult to distinguish from cardiac tumors. The eustachian valve ( Fig. 21-1) is an incomplete valve at the orifice of the inferior vena cava (IVC). The Chiari network is a remnant of congenital fibers that attach from the crista terminalis region to the eustachian valve. They are often mobile and may entrap a catheter or wire. The Chiari network does not typically prolapse through the tricuspid valve. The crista terminalis is a muscle ridge that extends from the right sides of the superior vena cava (SVC) and IVC and continues cephalad to the right atrial appendage. 1 The moderator band is a normal structure in the right ventricle that extends from the anterior papillary muscle to the septum and is a part of the septomarginal trabeculation. 2 The moderator band contains the right bundle branch conduction tissue. It is reported that Leonardo da Vinci first described this structure, calling it the “catena of the right ventricle.” 3 Another potential confounder is a left atrial cord or free fibrous cord. Left atrial cords have been well recognized in autopsy specimens and should not be confused with a true cor triatriatum. There is one description in the literature from an autopsy case describing a cord that originated from the right atrial wall and passed through a patent foramen ovale (PFO), attached to the left atrial wall and then the mitral valve, looped up and attached to the aortic valve, and finally to the aorta. 4 Prominent papillary muscles in either the right or left ventricles may appear to be intracardiac masses in some scan planes. Similarly, apical hypertrophic cardiomyopathy 5 or prominent ventricular noncompaction 6 can appear similar to cardiac tumors. Prominent apical trabeculation or a left ventricular false tendon can also appear to be an intracardiac mass or thrombus. 7 Figure 21-1 The eustachian valve is an embryologic remnant seen at junction of inferior vena cava and right atrium (RA). It may be misidentified as mass or thrombus. LA, Left atrium. Atrial findings that can be confused with cardiac tumors include the ridge of tissue between the upper pulmonary vein and left atrial appendage ( Fig. 21-2) and suture lines in the left atrium that are associated with cardiac transplantation. A prominent atrial septal aneurysm may be interpreted as a bulging cystic mass.8–10 Fatty infiltrate of the atrial septum, known as lipomatous atrial septal hypertrophy (LASH),11,12 can appear to be a cardiac tumor. LASH is a nonencapsulated hyperplastic accumulation of mature adipose tissue in the atrial septum ( Fig. 21-3, Video 21-1 Figure 21-2 The ridge of tissue between left upper pulmonary vein and left atrial appendage can be misinterpreted as mass or thrombus. Careful imaging will allow correct identification of this structure. LA, Left atrium; LV, left ventricle. Figure 21-3 Lipomatous atrial septal hypertrophy may be misinterpreted as tumor in atrial septum. LA, Left atrium; RA, right atrium. Vegetations are a type of intracardiac mass that must be distinguished from cardiac tumors. These are discussed in Chapter 20. Redundancy of the mitral valve supporting apparatus can appear to be an intracardiac mass, as can mitral annular calcification. Excess epicardial fat will appear to be an extracardiac mass; this has been associated with prolonged corticosteroid therapy. 15 A cardiac varix can occur in the right atrium16,17 and may appear to be a cardiac mass when seen on transesophageal echocardiographic (TEE) examination. 18 Similarly, fat in the atrioventricular (AV) groove or transverse sinus may be misinterpreted as an extracardiac mass. Several imaging artifacts can give rise to the appearance of cardiac masses, including beam-width artifact, side-lobe artifact, reverberation artifact, and mirror-image artifact (see Chapter 6). Fibrin debris in pericardial fluid can appear to be extracardiac masses. Pectus excavatum may displace structures anterior to the heart, giving rise to the appearance of an extracardiac mass. 19 Finally, a hiatal hernia can be confused as a mass when imaged from either transthoracic or transesophageal windows.20–22 This pseudo-mass will opacify when the patient ingests a carbonated beverage. In assessing masses by echocardiography, the size, location, mobility, attachment, and associated clinical history are important in establishing a differential diagnosis. 23 Cardiac tumors can be benign or malignant and primary or secondary.15,24–33 The most common primary cardiac tumors are summarized in Table 21-1. TABLE 21-1 The first premortem diagnosis of a cardiac tumor was made in 1934, when a sarcoma was identified. 34 Secondary (metastatic) tumors are up to 40 (or more) times more common than primary tumors. Of all cardiac tumors, 94% are benign and 6% are malignant. The most commonly encountered metastatic tumor at autopsy is lung carcinoma (17%). However, of malignancies, melanoma has the highest frequency of metastases to the heart (46%). According to Lam 26 in a series of 12,485 consecutive autopsies, there was an incidence of 1.23% (154) metastatic tumors and 0.056% (7) primary tumors. According to McAllister 35 in a series of 533 primary malignant cardiac tumors, angiosarcoma was the most common (31%), followed by rhabdomyosarcoma (21%), mesothelioma (15%), fibrosarcoma (11%), other sarcomas (9%), lymphoma (6%), and miscellaneous (7%). In a series of 319 primary benign cardiac tumors, 35 myxoma was the most common (40%), followed by lipoma (14%), papillary fibroelastoma (14%), rhabdomyoma (11%), fibroma (5%), hemangioma (5%), teratoma (4%), and other. There is a difference with age; rhabdomyoma is the most common tumor in pediatric patients, in whom this tumor type has a strong association with tuberous sclerosis. Masses usually found on heart valves are summarized in Table 21-2. TABLE 21-2 Masses found in the AV groove or pericardium may be interpreted as being in the heart. These include pericardial cysts, which are benign masses often incidentally found with echocardiography and typically located in the right AV groove, echinococcal (hydatid) cysts, coronary artery aneurysm ( Fig. 21-4), lipoma, pheochromocytoma, loculated pericardial effusion, or diaphragmatic hernia. Esophageal hiatal hernia has been misinterpreted as a cardiac mass during TEE. Abnormalities of the aorta may appear as intracardiac ( Fig. 21-5, Videos 21-2 and 21-3 Figure 21-4 Coronary artery aneurysms can appear in the atrioventricular (AV) groove. In this transthoracic echocardiogram example, the coronary artery aneurysm has calcified and appears as a solid mass. Subcostal transthoracic view shows calcified coronary artery aneurysm distorting right atrium. Figure 21-5 Abnormalities of the aorta may appear to be intra- or extracardiac masses. A, This atypical sinus of Valsalva aneurysm can be seen protruding into right atrium (RA) and right ventricle (RV), giving the appearance of a cystic mass. B, Color flow Doppler demonstrates flow from ascending aorta into aneurysm, giving rise to the appearance of a cystic mass in RA. LA, Left atrium. The cell of origin of the myxoma is still unclear.15,36–43 The most common location for myxomas (the other acceptable plural form is myxomata) is the left atrium, and the most common attachment is the atrial septum ( Fig. 21-6, Video 21-4 Figure 21-6 The most common site of myxoma attachment is left atrial side of atrial septum on fossa ovalis. A, Large smooth myxoma attached to left atrial side of atrial septum. B, Large pedunculated myxoma attached to left atrial side of atrial septum. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Figure 21-7 Large irregular myxoma attached to and moving with tricuspid valve. A, In diastole, myxoma prolapses into right ventricle, obstructing ventricular filling, as demonstrated by color flow Doppler imaging (B). Figure 21-8 Photograph of myxoma specimen removed at surgery, demonstrating irregular surface of tumor. Suture indicates point at which this myxoma attached to valve leaflet. Figure 21-9 Highly mobile myxoma is seen to move between left atrium and left ventricle during cardiac cycle. Surface shape may change as myxoma moves. A, Diastole. B, Systole. A highly mobile myxoma is more likely to be associated with embolization. Myxomas generally present between the ages of 30 to 60 years and may be spontaneous/sporadic, familial, or complex. Complexes that include myxoma may be referred to as syndrome myxoma, Carney syndrome, NAME syndrome (nevi, atrial myxoma, neurofibroma, ephelides), or LAMB syndrome (lentigines, atrial myxoma, blue nevi). Spontaneous (sporadic) myxomas are the most common; 70% are found in women.15,44 The familial variety 45 represents approximately 7% to 10% of myxomas, with autosomal dominant inheritance, and presents earlier in life (mean age 25). The familial form may be part of a complex syndrome that frequently presents with multiple tumors in several chambers. These are often in atypical locations (13% in the ventricular cavity), and patients require ongoing screening for a high recurrence rate that may approach 21% (usually occurring within 4 years after the initial resection). If a patient has multiple tumors, first-degree relatives should be screened. The Carney complex syndrome46–50 is uncommon and includes myxomas of skin, mucosa, or heart. Family history will be positive because these patients have an abnormal chromosome sequence at 17q22-24 encoding the regulatory subunit 1A of protein kinase A (PRKAR1A).51–54 There is an association with lentigenes, blue nevus, acromegaly, Cushing syndrome, thyroid cancer or nodules, melanotic schwannoma, breast ductal adenomas, and testicular tumors. The typical clinical presentation in patients with myxoma includes syncope, dyspnea, heart failure, and possibly sudden cardiac death, which is related to obstruction of the AV valve by the myxoma. There is embolization in approximately 30% to 40% of patients. Patients with myxoma often have fever, malaise, rash, weight loss, Raynaud phenomena, leukocytosis, thrombocytopenia, increased gamma globulins, anemia, and elevated C-reactive protein and erythrocyte sedimentation rate—all of which are nonspecific constitutional findings. Many of the symptoms associated with myxoma are thought to be due to synthesis and secretion of interleukin (IL)-6, since increased levels have been found in myxoma tissue. IL-6 is a proinflammatory cytokine that creates an acute-phase response. Papillary fibroelastoma (PFE) are generally small (mean 8 mm; up to 40 mm), usually single (>90%), typically attached to valve surfaces, and may be pedunculated and mobile.55–59 A study from the Cleveland Clinic 60 reported on a series of 162 patients with papillary fibroelastoma. Of these, 45% (49/162) were on the aortic valve, most often the right coronary cusp, followed by the noncoronary cusp, and least often on the left coronary cusp. Forty of 49 were on the aortic side of the aortic valve. The mitral valve was the location of 40/162 of these tumors: 23 anterior and 17 posterior leaflet; 32 of these 40 were on the atrial surface, and some attached to the supporting apparatus. Valve dysfunction or regurgitation related to PFE is uncommon. A PFE is generally round, oval, or irregular but homogeneous with well-demarcated borders. In approximately 50%, the PFE is attached via a stalk (50%) and is mobile. Embolic events secondary to PFE have been described,61,62 with resulting stroke, transient ischemic attack (TIA), angina, sudden death, myocardial infarction (MI), pulmonary embolism, and retinal artery occlusion. PFEs smaller than 2 mm will not be visible by TTE. PFE may be difficult to differentiate from degenerative valve disease. Surgical excision may be indicated in patients with large, mobile, left-sided tumors (>1 cm), those who have had embolic events, and those in whom complications are directly related to tumor mobility, such as coronary ostial occlusion.56,63 Tumor mobility is an independent predictor of death or nonfatal embolization, and in these patients, surgical intervention should be considered. Patients who are not surgical candidates may be given oral anticoagulants, but there are no randomized controlled data to support this. Rhabdomyoma is the most common cardiac tumor in infants and children.64–69 There has been some discussion about whether this is a true neoplasm or a hamartoma. Rhabdomyomas are usually multiple, involve both the left and right ventricular myocardium equally, generally project into the cavity, and may move freely (with some reported cases of outflow obstruction).70,71 In certain instances of outflow obstruction, surgical intervention is indicated, but spontaneous resolution of these tumors has been described, and surgical intervention is not generally necessary in the asymptomatic patient. There is an associated increased incidence of ventricular pre-excitation or Wolff-Parkinson-White syndrome in patients with rhabdomyoma. Studies have reported that many patients (30%-80%) with rhabdomyoma have associated tuberous sclerosis (angiofibromas on the face, fibromas around fingernails, café au lait spots, and subcutaneous nodules). 72 Fibromas are generally intramural within the left ventricular wall and occur mostly in infants and children.73–75 These tumors are histologically benign, but they may be associated with malignant dysrhythmias 76 and severe heart failure. It is estimated that 70% of patients with fibromas are symptomatic. If present, symptoms are related to obstruction, systolic dysfunction, or conduction abnormalities. Sudden death has been reported in approximately 15% of patients. Cardiac fibromas may appear as disproportional irregular hypertrophy and can be confused with thrombus or true hypertrophy. Surgical resection may help, but because these tumors may infiltrate extensively, resection will not necessarily ameliorate the problem. Sarcomas are the most common primary malignant cardiac tumors. They commonly occur between the ages of 30 and 50 years and are unusual in children. Sarcomas occur equally in men and women and are more common in the right heart chambers. There have been some reports of sarcomas developing around Dacron grafts or prosthetic valves in the heart and in peripheral vessels.77–79 A rat study published in 1958 suggested that plastics with long-chain polymers are carcinogenic with a long latency period. 80 More recently, it has been suggested that this may be related to reactive oxygen species. 81 Cardiac sarcomas are aggressive and associated with a rapid downhill clinical course. Death is related to widespread myocardial infiltration or extensive metastatic disease. Sarcomas may cause inflow or outflow obstruction and may invade into the pericardial space. Dysrhythmias are common. Metastatic tumors to the heart are more commonly carcinomas than sarcomas, simply because carcinomas are far more common than sarcomas. Secondary cardiac masses can reach the heart as a result of several mechanisms, which are summarized in Table 21-3. TABLE 21-3 The metastatic tumor can appear as discrete areas of enlargement in the myocardium or can present as extensive infiltration ( Fig. 21-10, Videos 21-6 and 21-7 Figure 21-10 Metastatic tumor invading atrial septum. A, Large tumor mass nearly fills right atrium (RA). B, Color flow Doppler demonstrates flow obstruction caused by large infiltrative mass in atria. LA, Left atrium; LV, left ventricle; RV, right ventricle; SVC, superior vena cava. Figure 21-11 Transthoracic echocardiogram done to evaluate possible tumor extension from liver into heart. A, Large tumor mass nearly fills right atrium (RA). Imaging demonstrated mass extending from hepatic vein into inferior vena cava and into RA. B, Flow from RA to right ventricle (RV) is impeded by large tumor mass in atrium. LA, Left atrium; LV, left ventricle. TEE is highly sensitive for detecting intracardiac thrombus or embolus and superior to transthoracic echocardiography (TTE) for evaluating many patients with intracardiac masses or thrombi.83–86 In one report of 231 patients with no preexisting indication for anticoagulation but with a recent TIA or stroke, 87 TTE and TEE were used to evaluate for a cardiac source of embolus. Over half (127) of these patients had a potential cardiac source demonstrated, but the source of embolus was demonstrated only by TEE in 90 of the 127 patients. Thrombus can form in any cardiac chamber or vessel under certain conditions, generally associated with abnormal flow dynamics. Echocardiographic characteristics of intracardiac thrombi include distinct margins and ultrasound reflection with motion that is independent of surrounding structures and visible in more than one imaging plane.88–91 Intracardiac thrombus is more frequently associated with abnormalities such as atrial fibrillation or flutter, mitral stenosis, and left ventricular dysfunction or acute MI. Intracardiac thrombus is also associated with foreign materials such as venous catheters ( Fig. 21-12), pacemaker leads, and surgery-related materials such as patches or sutures. Figure 21-12 Midesophageal transesophageal view demonstrating thrombus attached to a central venous catheter in right atrium. Development of intraventricular thrombus is more common following acute MIs of the anterior wall (compared with other regions)92–94 and is associated with worse outcome. 92 This appears to be true even with earlier and better percutaneous revascularization and platelet inhibitor therapy.95,96 Thrombus in the left ventricular apex is often better seen from transthoracic imaging windows ( Fig. 21-13, Video 21-8 Figure 21-13 Transthoracic echocardiography often provides superior imaging of left ventricular apex. Large thrombus is seen in apex in a region of hypokinesis. Colorization may improve ability to differentiate between ventricular muscle and attached thrombus. LV, Left ventricle. Figure 21-14 Transthoracic echocardiography often provides superior imaging of left ventricular apex. Multiple transthoracic echocardiography windows may be needed to clearly delineate size of an apical left ventricular thrombus. Figure 21-15 Transthoracic echocardiogram demonstrating presence of a large apical thrombus. Importance of thorough scanning is highlighted by this case, since usual mid-ventricular scan plane did not clearly demonstrate thrombus. Posterior tilt of transducer allowed visualization of large apical thrombus. LV, Left ventricle; RV, right ventricle. Thrombus can develop in the right atrium ( Fig. 21-16) and may be associated with indwelling catheters or pacemaker leads. Left atrial thrombus is more commonly found in patients with atrial fibrillation or mitral stenosis, and less commonly in patients with mitral regurgitation. Thrombus can be difficult to differentiate from other intracardiac masses, such as myxoma,85,105–107 or inversion of the left atrial appendage. 14
Imaging of Cardiac Tumors and Solid and Gaseous Materials
![]() Cardiac Pseudo-masses
Cardiac Pseudo-masses
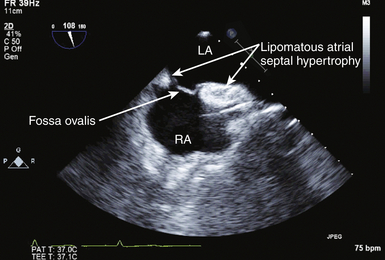
![]() ). These infiltrates can be very large (up to 7 cm or more), are common in elderly obese women, and have a possible association with atrial dysrhythmias. LASH creates a dumbbell-type appearance of the atrial septum due to sparing of the fossa ovalis, with fatty infiltrate in the inferior and superior portions of the atrial septum. LASH is typically preferential to the right atrium. When there is massive infiltration of the atrial septum, the adipose tissue in other parts of the heart is generally increased, particularly the right ventricular epicardial surface. Magnetic resonance imaging (MRI) is helpful in characterization of this abnormality if there is a clinical doubt after echocardiographic imaging. Adipose tissue has typical MRI characteristics that allow distinction from tumors or thrombus. There is no absolute diagnostic dimension established for the diagnosis of LASH, but 20 mm is often quoted. A dilated coronary sinus can bulge into the atrium as an extracardiac mass. Patients with prominent dilation of the coronary sinus should be evaluated for the presence of persistent left SVC-to–coronary sinus connection and for conditions associated with chronic elevation of right atrial pressure. Pectinate muscles in the right and left atrial appendages may be difficult to distinguish from tumor or thrombus. Similarly, a multilobed atrial appendage or an inverted left atrial appendage13,14 can be misinterpreted as cardiac masses.
). These infiltrates can be very large (up to 7 cm or more), are common in elderly obese women, and have a possible association with atrial dysrhythmias. LASH creates a dumbbell-type appearance of the atrial septum due to sparing of the fossa ovalis, with fatty infiltrate in the inferior and superior portions of the atrial septum. LASH is typically preferential to the right atrium. When there is massive infiltration of the atrial septum, the adipose tissue in other parts of the heart is generally increased, particularly the right ventricular epicardial surface. Magnetic resonance imaging (MRI) is helpful in characterization of this abnormality if there is a clinical doubt after echocardiographic imaging. Adipose tissue has typical MRI characteristics that allow distinction from tumors or thrombus. There is no absolute diagnostic dimension established for the diagnosis of LASH, but 20 mm is often quoted. A dilated coronary sinus can bulge into the atrium as an extracardiac mass. Patients with prominent dilation of the coronary sinus should be evaluated for the presence of persistent left SVC-to–coronary sinus connection and for conditions associated with chronic elevation of right atrial pressure. Pectinate muscles in the right and left atrial appendages may be difficult to distinguish from tumor or thrombus. Similarly, a multilobed atrial appendage or an inverted left atrial appendage13,14 can be misinterpreted as cardiac masses.
![]() Imaging True Cardiac Masses
Imaging True Cardiac Masses
Malignant Primary Cardiac Tumors
Benign Primary Cardiac Tumors
Angiosarcoma
Rhabdomyosarcoma
Mesothelioma
Fibrosarcoma
Leiomyosarcoma
Synovial sarcoma
Lymphoma
Myxoma
Lipoma
Papillary fibroelastoma
Rhabdomyoma
Fibroma
Hemangioma
Teratoma
Mesothelioma of atrioventricular node
Mass Found on Valve
Comments
Papillary fibroelastoma
Pedunculated, mobile
Lambl excrescence
Thin, mobile filamentous structures; single or multiple; 1 mm thick; 1-5 mm long; along closure line of valve, AV>>MV; avascular connective tissue matrix covered by single layer of endothelium; degenerative process; frequency increased with age
Fenestration
Degenerative change in valve leaflet
Cysts
Blood cyst is thin walled; multilobed; attached to valve leaflet
Immune-mediated valve disease
Nodules of Arantius and lunulae
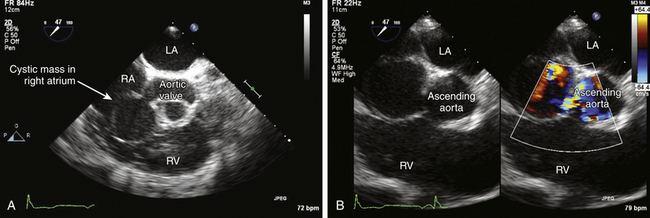
![]() ) or extracardiac masses.
) or extracardiac masses.
Primary Benign Cardiac Tumors
Myxoma
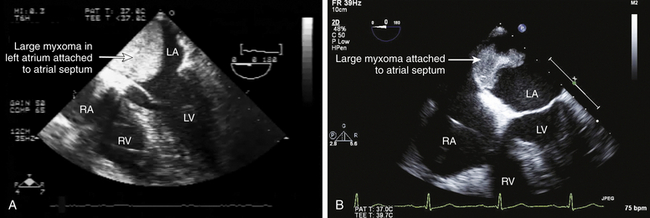
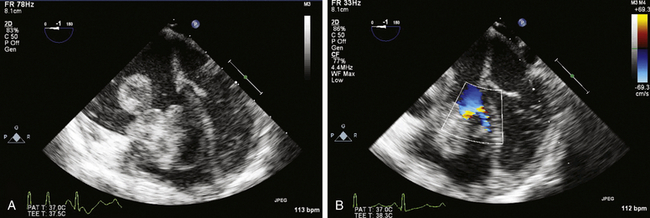
![]() ). The reported distribution of myxomas is 75% in the left atrium, 20% in the right atrium, and the remainder in the left or right ventricle. There are very rare case reports of myxomas on the AV valves ( Fig. 21-7, Video 21-5
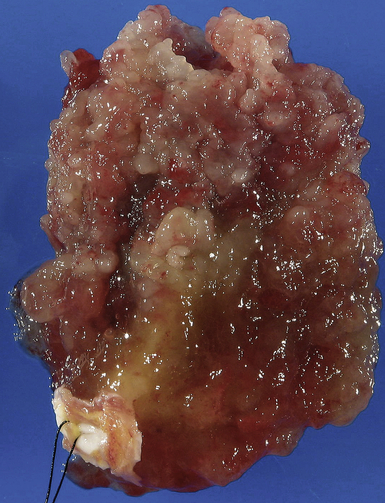
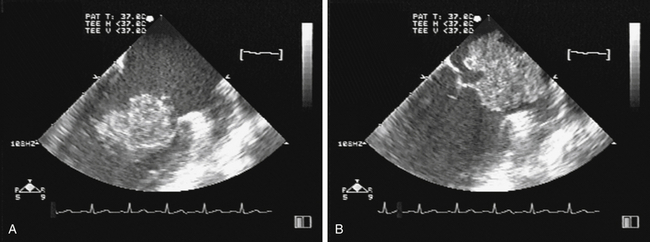
). The reported distribution of myxomas is 75% in the left atrium, 20% in the right atrium, and the remainder in the left or right ventricle. There are very rare case reports of myxomas on the AV valves ( Fig. 21-7, Video 21-5 ![]() ). Myxomas may be smooth or papillary or grapelike in appearance ( Fig. 21-8); these myxomas are more likely to embolize than well-encapsulated tumors. Typically, myxomas are pedunculated (although they may be sessile) and attached via a narrow stalk in the fossa ovalis region. Myxomas appear heterogeneous and may have cavitations and protruding frond-like extensions. The myxoma tumor may be large enough to prolapse through the AV valve ( Fig. 21-9), causing obstruction and the associated physical exam findings (diastolic murmur or “plop”). Color flow and continuous wave Doppler allow assessment of the degree of functional stenosis/flow obstruction.
). Myxomas may be smooth or papillary or grapelike in appearance ( Fig. 21-8); these myxomas are more likely to embolize than well-encapsulated tumors. Typically, myxomas are pedunculated (although they may be sessile) and attached via a narrow stalk in the fossa ovalis region. Myxomas appear heterogeneous and may have cavitations and protruding frond-like extensions. The myxoma tumor may be large enough to prolapse through the AV valve ( Fig. 21-9), causing obstruction and the associated physical exam findings (diastolic murmur or “plop”). Color flow and continuous wave Doppler allow assessment of the degree of functional stenosis/flow obstruction.
Papillary Fibroelastoma
Rhabdomyoma
Fibroma
Primary Cardiac Malignant Tumors
Sarcoma
Metastatic Cardiac Tumors
Mechanism
Tumor Types
Direct extension
Lung, breast, esophageal, mediastinal
Venous spread
Renal (hypernephroma) most common; up to 43% of patients with this tumor have right atrial involvement; 82 adrenal, hepatoma, thyroid, lung, leiomyosarcoma
Hematogenous
Melanoma (46%), breast, lung, genitourinary tract, gastrointestinal tract
Lymphatic
Leukemia, lymphoma
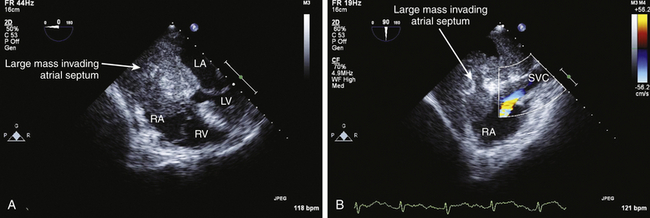
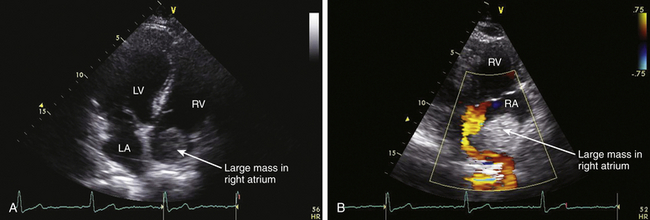
![]() ). Extension of renal or liver malignancy via the IVC can sometimes be imaged using echocardiography ( Fig. 21-11). Cardiac metastases should be suspected when a patient with a primary tumor in an organ other than the heart develops cardiac enlargement, dysrhythmias, or heart failure. However, only a minority (10%) of patients with cardiac metastases develop signs or symptoms of cardiac dysfunction. Of those that develop cardiac signs or symptoms, 90% result from pericardial involvement and 10% from intracavitary or intramyocardial involvement. 15 It is rare that the metastatic lesion will be limited to the heart: cardiac metastases usually indicate widespread metastases.
). Extension of renal or liver malignancy via the IVC can sometimes be imaged using echocardiography ( Fig. 21-11). Cardiac metastases should be suspected when a patient with a primary tumor in an organ other than the heart develops cardiac enlargement, dysrhythmias, or heart failure. However, only a minority (10%) of patients with cardiac metastases develop signs or symptoms of cardiac dysfunction. Of those that develop cardiac signs or symptoms, 90% result from pericardial involvement and 10% from intracavitary or intramyocardial involvement. 15 It is rare that the metastatic lesion will be limited to the heart: cardiac metastases usually indicate widespread metastases.
![]() Imaging Thrombi and Emboli
Imaging Thrombi and Emboli
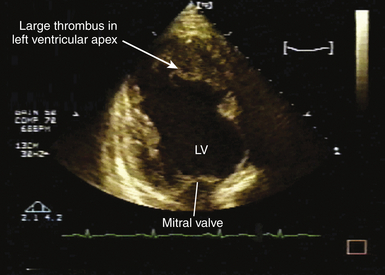
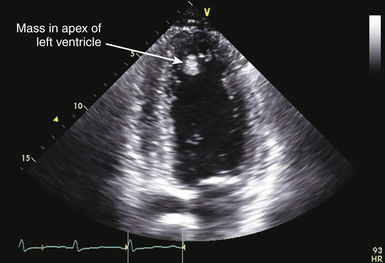
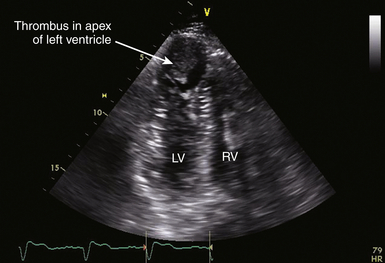
![]() ). In the setting of thrombus formation following MI, there will be abnormal wall motion in the region underlying the thrombus ( Fig. 21-14). There may be organization or calcification of a thrombus that has been present for some time following infarction ( Fig. 21-15). Thrombus may also develop in aneurysmal regions of the left ventricle. Ventricular thrombus may also develop in dilated cardiomyopathy (see Chapter 18). Patients with left ventricular thrombus are at increased risk for embolic events and death.84,92 It may be difficult to identify mural thrombus in some patients, although use of echocardiographic contrast agents is reported to improve diagnostic sensitivity.97–100 Similarly, three-dimensional (3D) echocardiography101–103 is reported to improve detection of mural thrombi and allow better assessment of thrombus mobility and volume. Intraoperative TEE can allow diagnosis of mural thrombi following acute MI, which may lead to important alterations of patient management. 104
). In the setting of thrombus formation following MI, there will be abnormal wall motion in the region underlying the thrombus ( Fig. 21-14). There may be organization or calcification of a thrombus that has been present for some time following infarction ( Fig. 21-15). Thrombus may also develop in aneurysmal regions of the left ventricle. Ventricular thrombus may also develop in dilated cardiomyopathy (see Chapter 18). Patients with left ventricular thrombus are at increased risk for embolic events and death.84,92 It may be difficult to identify mural thrombus in some patients, although use of echocardiographic contrast agents is reported to improve diagnostic sensitivity.97–100 Similarly, three-dimensional (3D) echocardiography101–103 is reported to improve detection of mural thrombi and allow better assessment of thrombus mobility and volume. Intraoperative TEE can allow diagnosis of mural thrombi following acute MI, which may lead to important alterations of patient management. 104
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Thoracic Key
Fastest Thoracic Insight Engine
