23
Echocardiographic Evaluation of Pericardial Disease
 Pericardial Anatomy and Physiology
Pericardial Anatomy and Physiology
Pericardial Anatomy
The simplest description of the pericardium is that of a bilayered sack surrounding the heart, its appendages, and its proximal vascular connections. The pericardium measures roughly 2 mm in thickness, and its two layers are separated by a potential space containing 5 to 50 mL of fluid under normal physiologic conditions. The translucent visceral pericardium comprises the inner layer and is in direct continuity with the epicardium at its basal lamina. The opaque thicker outer layer is termed the parietal pericardium. The two layers fuse together at various points where they form reflections that give rise to the pericardial space and the two pericardial sinuses. Pericardial reflections surrounding the venae cavae and pulmonary veins give rise to the oblique sinus, and the transverse sinus is formed by pericardial reflections around the proximal portions of the pulmonary artery and ascending aorta ( Fig. 23-1). The transverse pericardial sinus is frequently imaged with transesophageal echocardiography (TEE) in the midesophageal long-axis view ( Fig. 23-2).1,2
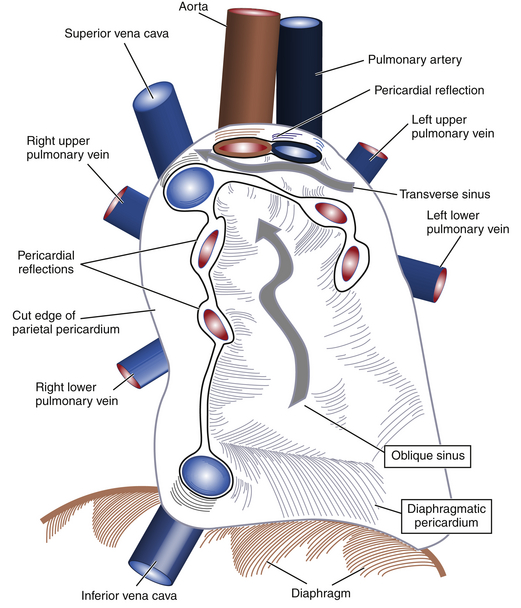
Figure 23-1 Schematic showing how visceral and parietal pericardial tissues meet to create reflections that give rise to pericardial space and two pericardial sinuses (wide gray arrows). (From Savage RM, Aronson S. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2010.)
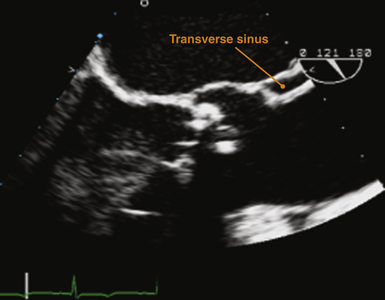
Figure 23-2 Transesophageal echocardiographic image obtained in midesophageal long-axis view. Orange marker indicates echolucency representing a small amount of pericardial fluid within transverse sinus just posterior to proximal ascending aorta. (From Savage RM, Aronson S. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2010.)
Each pericardial layer is composed of loose connective tissue and an interdigitated monolayer of serosal mesothelial cells, all of which have characteristic microvillous projections. The pericardial connective tissue contains nerves, arteries, lymph nodes, and lymphatics. The same mesothelial layer that is a portion of the visceral pericardium is continuous with the parietal pericardium. However, the parietal pericardium is distinct in that its outer covering contains the fibrosa, a grouping of fibrocollagenous tissue lending thickness, increased density, and strength to the pericardium ( Fig. 23-3). The microvilli-lined mesothelial cells produce a plasma ultrafiltrate that is the pericardial fluid.1,2
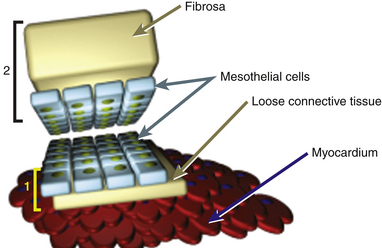
Figure 23-3 Schematic of gross composition and relationship of 1, visceral pericardial (yellow bracket) and 2, parietal pericardial (black bracket) tissues. Parietal pericardium is notably thicker owing to presence of fibrosa. (From Savage RM, Aronson S. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2010.)
Pericardial Microphysiology
Reorganization and hypertrophy of mesothelial cells permits slow accumulation of a relatively large volume of fluid in the pericardial space. However, rapid accumulation of fluid on the order of minutes or hours to days does not allow adequate time for mesothelial reorganization, and tamponade physiology may occur in this clinical scenario. Pressure-volume curves with a characteristic “J” shape can be used to characterize both acute and chronic accumulation of pericardial fluid ( Fig. 23-4). 3
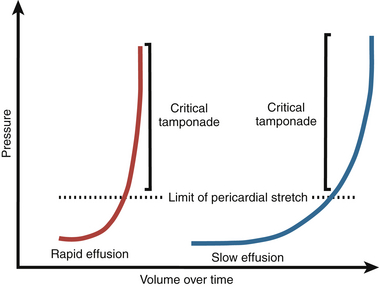
Figure 23-4 “J-shaped” pressure-volume curves represent temporal relationship between rapidly (red line) and slowly (blue line) accumulating pericardial fluid and accompanying simultaneous changes in developed intrapericardial pressure. (From Spodick DH. Acute cardiac tamponade. N Engl J Med. 2003;349:684-690.)
Pericardial Macrophysiology
The parietal pericardium is believed to contribute to maintaining the characteristic heart-shaped morphology of the organ. In individuals whose hearts have undergone pericardiectomy or pericardiotomy, the organ is commonly observed to assume a more globular shape, although cardiac function does not appear to change grossly in such individuals. Further, a number of tendinous intersections arise from the parietal pericardium that attach at various points within the mediastinum and serve to stabilize the heart in the chest during various movements of the body. The central tendon of the diaphragm coalesces with the diaphragmatic parietal pericardium to contribute to anchoring the organ in the mediastinum (see Fig. 23-1).1,2
Introducing and understanding the concept of respirophasic variation is a prerequisite to understanding how echocardiography can be used to evaluate pericardial physiology. Respirophasic variation is the term used to describe changes in blood flow within the heart in relation to pressure dynamics in the intrapleural and intrapericardial cavities. During normal spontaneous respiration, changes in intrapleural pressures are nearly equally transmitted to the pericardial space and intracardiac chambers; thus, the pericardial pressure typically approximates the intrapleural pressure and will vary with the respiratory cycle. Pericardial pressures are typically about −6 mmHg at end-inspiration and −3 mmHg at end-expiration. During spontaneous inspiration, it is the lowering of intrathoracic and pericardial pressures that augments venous return and allows increased filling of the right-sided chambers. Transmural pressure determines the true filling pressure of the heart and is calculated by subtracting the pericardial pressure from the intracardiac pressure ( Fig. 23-5, A). Negative inspiratory intrapleural pressure also causes blood pooling in the lower-compliance pulmonary venous circulation, which in turn causes a reduction in left atrial (LA) filling. The decrease in LA venous return causes a drop in both left ventricular (LV) stroke volume and cardiac output. In addition, the drop in intrathoracic pressure during spontaneous inspiration in relation to the higher extrathoracic systemic arterial pressures results in a reduction of left heart output due to a relatively higher afterload secondary to the negative intrapleural pressure ( Fig. 23-5, B). Changes in systemic vascular resistance are the primary determinants of LV afterload, but the relative differences between intrapericardial and intrathoracic pressures do affect this parameter. Respiratory cycle–related intrapericardial pressure dynamics are reflected in Doppler assessments of changes of the transatrioventricular valvular velocities. During the inspiratory phase of spontaneous respiration, transtricuspid velocities typically increase by 20% ( Fig. 23-6, A). Conversely, transmitral velocities typically decrease by roughly 10% during spontaneous inspiration ( Fig. 23-6, B).
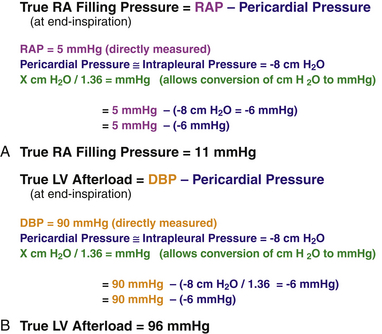
Figure 23-5 Sample calculations that permit determination of (A) true filling pressure of right heart and (B) true left ventricular afterload. (From Savage RM, Aronson S. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2010.)
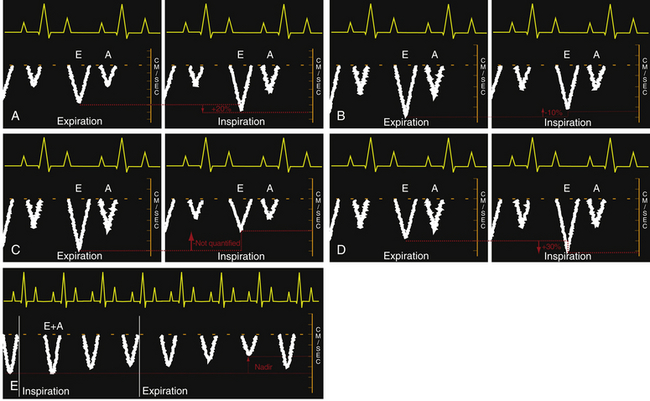
Figure 23-6 Idealized transesophageal echocardiographic imaging of transtricuspid pulsed wave Doppler (PWD) profiles during (A) spontaneous respiration and (C) intermittent positive pressure ventilation (IPPV). Idealized transmitral PWD tracings are also presented for (B) spontaneous respiration, (D) IPPV, and (E) intermittent positive pressure in a hypovolemic patient. A, Late diastolic filling associated with atrial contraction; E, early diastolic ventricular filing; E+A, fused waveforms of the early (E) and late (A) diastolic filling velocities. (From Savage RM, Aronson S. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2010.)
Opposite changes in these transvalvular velocities are produced by the use of intermittent positive pressure ventilation (IPPV). The increased pericardial pressure that accompanies IPPV results in a decrease in both right heart filling and output which is reflected in the observed decreased transtricuspid diastolic velocities. The magnitude of the relative decrease in transtricuspid velocities during IPPV will depend on the amount of positive pressure transmitted to the right heart as well as the intravascular volume status ( Fig. 23-6, C). The rise in intrathoracic pressure in relation to extrathoracic systemic pressure during IPPV inspiration leads to an increase in left heart output. In addition, increased intrathoracic pressure during IPPV propels blood from the lower-compliance pulmonary veins into the LA and LV, boosting left heart output. The increase in left heart inflow is manifested as an observed increase of approximately 30% in transmitral valvular velocities ( Fig. 23-6, D). An alternative pattern of transmitral flow has been observed in a canine model that is characterized by a decrease in transmitral flow velocities, which reach the nadir during expiration ( Fig. 23-6, E). The authors of this work attributed these unexpected findings to a significant degree of intravascular depletion. Respirophasic variation is the term used to describe these changes in transatrioventricular valvular velocities.1,4
Physiologic variables such as age, heart rate, rhythm, preload, volume flow rate, ventricular systolic function, diastolic function, and atrial contractility all contribute to the observed velocities. Certain pericardial pathologies may blunt transmission of intrathoracic pressures to intrapericardial structures (e.g., pericarditis or pericardial tamponade), leading to reduced transatrioventricular velocities that are often observed under these clinical conditions.1,2,4
 Pericardial Pathology
Pericardial Pathology
Evaluation of pericardial disease is performed with echocardiography to rule out specific pathologies that can directly impact myocardial function. A multiwindow, multiplane TEE or transthoracic echocardiographic (TTE) examination is necessary to thoroughly evaluate the pericardium. Box 23-1 presents the five basic categories of pericardial pathology. 1
Congenital Pericardial Defects
Congenital pericardial defects are exceedingly rare and may consist of partial or total absence of the pericardium.5,6 However, Mulibrey nanism, an autosomal recessive disorder primarily found in the Finnish population, is a congenital pericardial disorder that does not involve any degree of pericardial absence. Instead, it is partially characterized by development of constrictive pericarditis (CP) in later life among some afflicted patients. If the degree of CP becomes significant, these individuals can present with diastolic heart failure. CP is discussed in a later section. 7
Most patients with any degree of pericardial absence are clinically asymptomatic ( Table 23-1). 8 One small observational study found that paroxysmal stabbing chest pain that mimicked coronary ischemia was the most common clinical presentation. The putative mechanism for the observed chest pain is a temporary kinking and obstruction of coronary vessels local to the pericardial defect as a portion of the myocardium herniates through the defect. Patients with congenital absence of the pericardium are at increased risk for additional congenital abnormalities in that up to 30% of these patients will have other pathology (e.g., septal defects). 9
TABLE 23-1
Pathologic Characteristics of Forms of Pericardial Absence
| Form | Incidence ∗ | Clinical Significance |
| Total bilateral absence | Rare | Mostly asymptomatic |
| Partial left absence | 70% | Increased risk for aortic dissection if augmented heart mobility; possible herniation/strangulation of heart structures through defect, with chest pain, shortness of breath, syncope |
| Partial right absence | 17% | Increased risk for aortic dissection if augmented mobility as a result of defect |
| Overall | 0.01% |
∗Incidence values represent a percentage of the overall incidence of 0.01%.
Using echocardiography, the most common findings associated with partial pericardial absence are cardiac hypermobility, paradoxical motion or flattening of the ventricular septum during systole, an exaggerated posterior LV wall, and the possible appearance of a dilated right ventricle (RV) together with a reduced dimension of the right atrium (RA). Cardiac magnetic resonance imaging (MRI), computed tomography (CT), and plain chest films (to a lesser extent) are the imaging modalities most useful in making the definitive diagnosis of congenital absence of the pericardium ( Fig. 23-7). Electrocardiographic (ECG) findings in these patients may include bradycardia, right bundle branch block, poor R-wave progression in the precordial transition leads, and prominent P waves in the mid-precordial leads. 9
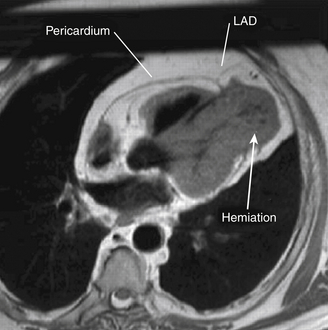
Figure 23-7 Cardiac magnetic resonance image showing herniation of ventricular myocardium (arrow). LAD, Left anterior descending coronary artery. (From Chassaing S, Bensouda C, Bar O, et al. A case of partial congenital absence of pericardium revealed by MRI. Circ Cardiovasc Imaging. 2010;3:632-634.)
Pericarditis
Pericarditis refers to inflammation of the pericardium due to various pathologies. It may present as either an acute or chronic process and is often associated with development of a pericardial effusion. Acute pericarditis may progress to chronic CP, which may induce severe diastolic dysfunction. The classic clinical triad of pericarditis consists of chest pain, diffuse ST-segment elevations on ECG, and a pericardial rub on auscultation. Table 23-2 lists the various clinical etiologies of pericarditis. 8
TABLE 23-2
Clinical Disorders Related to Development of Pericarditis
| Category | Detailed Causes |
| Immune/inflammation related | Rheumatoid arthritis, systemic lupus erythematosus, acute rheumatic fever, dermatomyositis, Wegener granulomatosis, mixed collagen vascular disease, post myocardial infarction (Dressler syndrome), uremia, post cardiac surgery (inflammation related) |
| Infection related | Bacterial infections (e.g., tuberculosis), post viral |
| Neoplasm related | Primary mesothelioma, fibrosarcoma, secondary metastatic disease (e.g., melanoma, lymphoma, leukemia, or direct extension of pulmonic or breast tumor) |
| Intracardiac/pericardial communication related | Chest trauma, post percutaneous catheter interventional procedures (e.g., cardiac valve replacement or valvulopathy, coronary interventions, arrhythmia-related procedures) |
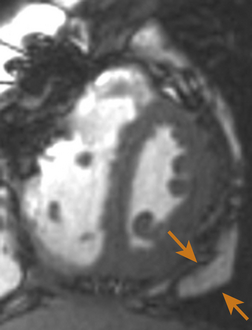
Echocardiographically, the pericardium is inspected for any evidence of thickening. This typically appears as an increase in brightness (echogenicity) of the ultrasound signal. Normal pericardial thickness is approximately 2 to 3 mm. M-mode may prove useful to discern and quantify any degree of pericardial thickening; it reveals multiple parallel ultrasound reflections ( Fig. 23-8). Multiple echo windows must be obtained to verify the diffuse or localized nature of pericarditis. Unfortunately, echocardiography lacks adequate fidelity to consistently quantify the degree and anatomic extent of pericarditis and thus has inadequate sensitivity and specificity for detecting the disorder when compared to other higher-fidelity imaging modalities (e.g., cardiac MRI, 64-slice CT). Figure 23-9 presents an MRI image of focally thickened pericardium.
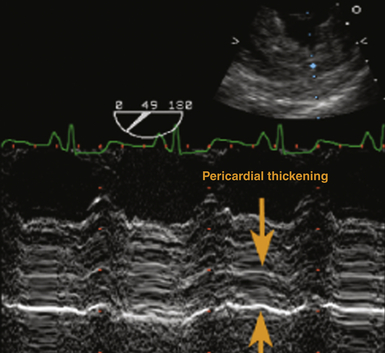
Figure 23-8 Transesophageal echocardiographic image, transgastric short-axis view using M-mode. Severely thickened pericardium is denoted between orange arrowheads. (From Savage RM, Aronson S. Comprehensive Textbook of Perioperative Transesophageal Echocardiography. Philadelphia: Lippincott Williams & Wilkins; 2010.)