19
Aneurysms and Dissections
 Thoracic Aorta
Thoracic Aorta
The thoracic aorta can be comprehensively evaluated by transesophageal echocardiography (TEE). 1 Aneurysms and dissections of the aorta produce structural lesions that are readily detectable by TEE in high resolution, owing to the close anatomic relationship between the esophagus and aorta. This is limited, however, in the distal ascending aorta and proximal aortic arch because the trachea lies between the esophagus and aorta, rendering TEE blind to these aortic segments. 2 This blind spot of TEE can be eliminated with epiaortic imaging, which together with epicardial imaging complements TEE for evaluation of aortic diseases.3–5
TEE is a high-quality imaging modality for aortic evaluation. 6 Firstly, TEE is indispensable for emergency evaluation of clinically unstable patients with acute aortic syndromes.6–7 Secondly, TEE, including three-dimensional (3D) imaging, can also detect complications of aortic dissection such as pericardial tamponade, aortic regurgitation, and myocardial ischemia. 8 Thirdly, TEE can guide surgical decision making before and after aortic intervention.6–8
Although TEE is feasible in awake patients, topical anesthesia and sedation must be adequate to prevent aortic rupture from hypertensive responses during TEE examination. 9 Although TEE is safe during general endotracheal anesthesia, giant aortic aneurysms may still render TEE hazardous. The risk of esophageal perforation during TEE examination may be higher due to extrinsic esophageal compression. 10 Furthermore, insertion of a TEE probe may aggravate pulmonary artery and/or major airway compression, precipitating severe hypoxemia from ventilation/perfusion mismatch.11,12
Anatomy of the Aorta
Aortic Segments
The aortic root begins at the ventricular-aortic junction and terminates at the sinotubular junction. The aortic root complex consists of the aortic valve annulus, aortic valve cusps, sinuses of Valsalva (sinus segment), and sinotubular junction, where the sinus segment joins the tubular ascending aorta. The aortic root and proximal ascending aorta lie within the pericardium. The weakest area of the aortic wall is the sinus segment. These anatomic relationships are important because aortic rupture at this level will be intrapericardial and cause acute cardiac tamponade. The aortic root components are clearly demonstrated by TEE in the midesophageal (ME) aortic valve long-axis view, where their diameters can be precisely quantified ( Fig. 19-1). 13 The aortic valve cusps and their corresponding sinuses of Valsalva are described by their anatomic relationship to the coronary artery ostia as follows: right coronary, left coronary, and noncoronary. The aortic root complex is also known as the functional aortic annulus, since alterations in one or more of its components can produce aortic regurgitation. 13
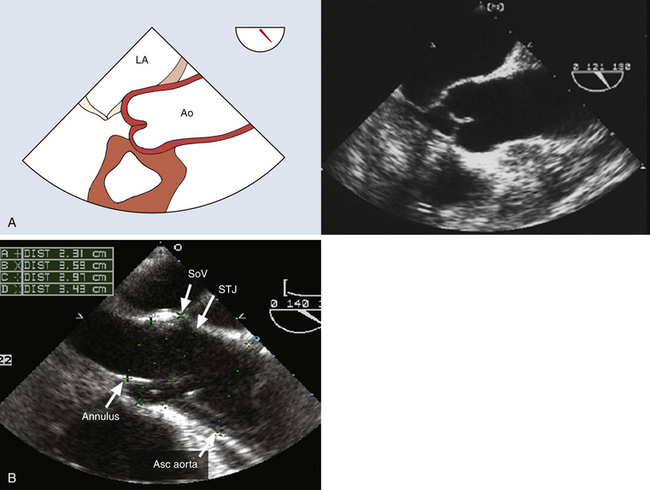
Figure 19-1 A, Midesophageal (ME) aortic valve long-axis view at a multiplane angle of 121 degrees. This view images aortic root in cross-section and is ideal for quantifying diameters of aortic root at the levels of aortic valve annulus, sinuses of Valsalva, and sinotubular junction. B, ME aortic valve long-axis image at a multiplane angle of 140 degrees. Patient has a Stanford type A aortic dissection characterized by an intimal flap originating in aortic root and extending into ascending aorta (Asc aorta). Aortic diameters were quantified as follows: A, aortic valve annulus; B, sinuses of Valsalva; C, sinotubular junction; and D, ascending aorta. Ao, Aorta; LA, left atrium; SoV, sinuses of Valsalva; STJ, sinotubular junction. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
The ascending aorta is defined as the tubular aorta that ascends from the sinotubular junction to join the aortic arch at the origin of the innominate artery. The ascending aorta can be imaged in both short and long axis at the level of the right pulmonary artery ( Fig. 19-2). As explained earlier, TEE fails to interrogate the distal ascending aorta and proximal aortic arch owing to the attenuation of ultrasound imaging across the air-filled trachea. 2
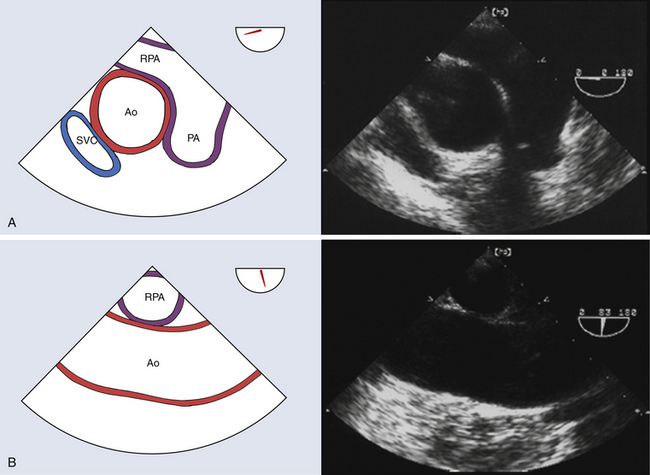
Figure 19-2 A, Midesophageal (ME) ascending aortic short-axis view with a multiplane angle in the 30-degree range. Ascending aorta can be clearly assessed for dissection and aneurysm. This view provides the opportunity to measure ascending aortic diameter at level of right pulmonary artery. B, ME ascending aortic long-axis view at a multiplane angle of 83 degrees. This view facilitates analysis of ascending aorta at level of right pulmonary artery with regard to dimensions, dissection, aneurysm, atheroma, calcification, and rupture. Ao, Aorta; PA, main pulmonary artery; RPA, right pulmonary artery; SVC, superior vena cava. (See Video 19-1. ![]() ) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
The aortic arch contains the origins of the brachiocephalic vessels—the innominate, left carotid, and left subclavian arteries ( Fig. 19-3). Anatomic variations in aortic arch branch vessel anatomy have a collective incidence as high as 25%. 14 The most frequent variant pattern (with a 20% incidence) is the bovine arch, defined as a common origin for the innominate and left common carotid arteries. 15
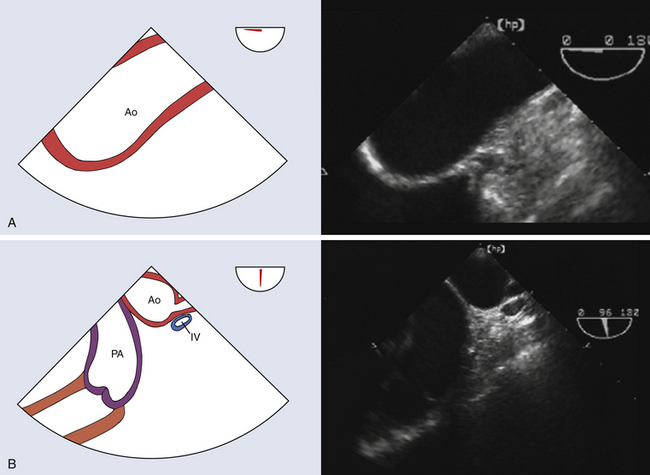
Figure 19-3 A, Upper esophageal (UE) aortic arch long-axis view at a multiplane angle of 0 degrees. This view interrogates distal aortic arch and facilitates detection of dissection and/or aneurysm in aortic arch. B, UE aortic arch short-axis view at a multiplane angle of 86 degrees. This view interrogates distal aortic arch and frequently images pulmonary artery, innominate vein, subclavian artery origin, and left carotid artery origin. This view facilitates detection of aortic arch branch involvement with dissection or aneurysm. Ao, Aorta; IV, innominate vein; PA, main pulmonary artery. (See Video 19-2. ![]() ) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
The descending thoracic aorta extends from the left subclavian artery to the diaphragmatic hiatus ( Fig. 19-4). Its major branches are the paired intercostal arteries that contribute substantially to the spinal cord collateral network. The aortic isthmus lies just beyond the origin of the left subclavian artery at the site of the ligamentum arteriosum, a vestige of the fetal ductus arteriosus. The aortic isthmus is the most common location for aortic coarctation, patent ductus arteriosus (PDA), and traumatic intimal disruptions.
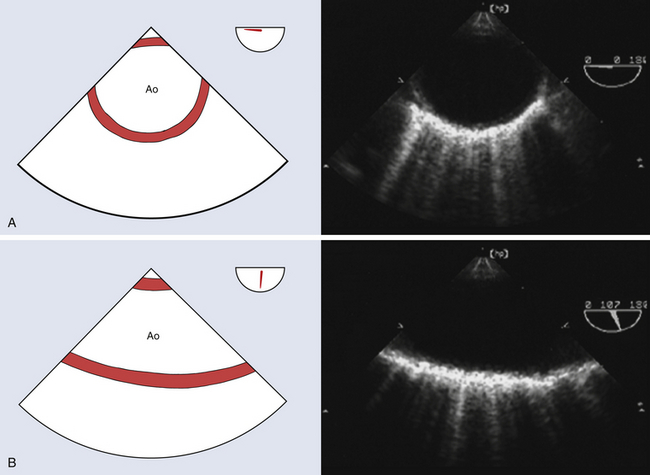
Figure 19-4 A, Midesophageal (ME) descending aortic short-axis view at a multiplane angle of 0 degrees. It facilitates determination of descending aortic diameter. Entire descending thoracic aorta can be evaluated for dissection and aneurysm by manipulating TEE probe along the length of this aortic segment. B, ME descending aortic long-axis view at a multiplane angle of 107 degrees. It provides an opportunity for detailed interrogation of intimal surface of descending thoracic aorta. Ao, Aorta. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Aortic Segmental Anatomy for Thoracic Endovascular Aortic Repair
Aortic Arch
The proximal thoracic aorta is divided into five anatomic zones that describe the landing zone for thoracic aortic endovascular repair (TEVAR) ( Fig. 19-5). 16 Zone 0 is defined as the ascending aorta and proximal aortic arch to the innominate artery. Zone 1 is defined as the aortic segment between the innominate and left carotid arteries. Zone 2 is defined as the aortic segment between the left common carotid artery and the left subclavian artery. Zone 3 is defined as the curved segment of the distal aortic arch and proximal descending thoracic aorta beyond the left subclavian artery. Zone 4 is defined as the straight part of the descending thoracic aorta from the level of the 4th thoracic vertebra.
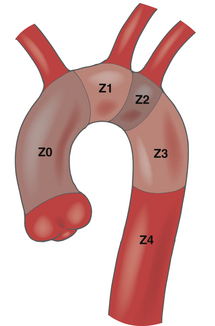
Figure 19-5 Aortic landing zones for thoracic endovascular aortic repair. Thoracic aorta is divided into five anatomic zones that relate to the landing zone for thoracic endovascular aortic repair. Zone 0, ascending aorta and proximal arch to innominate artery; Z1, segment between innominate artery and left common carotid artery; Z2, segment between left common carotid and left subclavian arteries; Z3, segment beyond left subclavian along curved portion of distal arch; Z4, straight portion of descending thoracic aorta starting at level of the 4th thoracic vertebra. Z, Zone. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Although zones 2 through 4 are accessible landing zones for TEVAR, a zone 2 landing requires left subclavian coverage that often prompts an ancillary vascular procedure to minimize the risks of stroke and upper extremity ischemia.17,18 Zones 0 and 1 landings require debranching of the brachiocephalic vessels prior to TEVAR to maintain cerebral perfusion. 19 These techniques are collectively termed hybrid aortic arch repair. 19
Descending Thoracic Aorta
The descending thoracic aorta has also been classified into three types of coverage with respect to TEVAR.20,21 Type A coverage extends from the left subclavian artery to the level of the 6th thoracic vertebra.20,21 Type B coverage extends from the level of the 6th thoracic vertebra to the diaphragmatic hiatus.20,21 Type C coverage extends from the left subclavian artery to the diaphragmatic hiatus.20,21
Important Anatomic Relationships of the Aorta
The anatomic relationships between the thoracic aorta and esophagus are clinically important ( Fig. 19-6). The aortic root and proximal ascending aorta lie within the pericardium, explaining the risk for cardiac tamponade in acute aortic dissection ( Fig. 19-7). Since these aortic segments also lie anterior to the esophagus and left atrium, they are clearly imaged by TEE, with the left atrium providing the acoustic window (see Fig. 19-1). The right pulmonary artery courses between the esophagus and ascending aorta, providing an acoustic window and anatomic reference for TEE imaging of the ascending aorta (see Figs. 19-1 and 19-2).
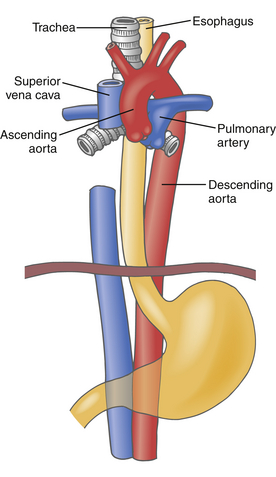
Figure 19-6 Diagram illustrating anatomic relationships between thoracic aorta (red), main pulmonary artery (blue), left pulmonary artery, right pulmonary artery, superior vena cava (blue), inferior vena cava, esophagus (yellow), and trachea (gray). Descending thoracic aorta is anterior to esophagus near aortic arch, then lateral to esophagus in mid-thorax, and finally posterior to esophagus at diaphragmatic hiatus. Distal trachea and left mainstem bronchus lie between esophagus and aortic arch. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
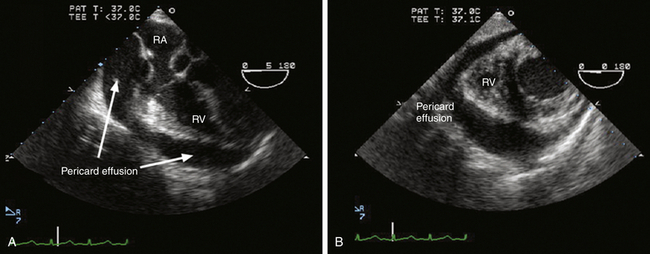
Figure 19-7 Aortic rupture with hemopericardium. This image pair demonstrate midesophageal four-chamber view (A) and transgastric ventricular short-axis view (B) in a hypotensive patient with Stanford type A aortic dissection. Circumferential pericardial effusion shown in both images was diagnostic for rupture of proximal ascending aorta causing hemopericardium. A demonstrates diastolic collapse of right atrium (RA), indicating development of pericardial tamponade. RV, Right ventricle. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Since the ascending aorta initially lies directly anterior to the esophagus, it is clearly visualized during TEE imaging. The distal trachea and left mainstem bronchus obstruct TEE imaging of the distal ascending aorta and proximal aortic arch because they course between the esophagus and these two aortic segments (see Fig. 19-6). The descending thoracic aorta is initially anterior to the esophagus, then lateral to the esophagus in the mid-thorax, then posterior to the esophagus at the diaphragmatic hiatus (see Fig. 19-6). Despite this changing anatomic relationship with the esophagus, the descending thoracic aorta can be imaged clearly by TEE throughout its entire length.
Normal Size of the Aorta
Although there are guidelines for the normal aortic segment dimensions, these normal values vary according to age, gender, and body size ( Table 19-1).6,22,23 The aortic diameter is greatest in the sinus segment and then tapers gradually beyond the sinotubular junction (see Table 19-1). Recent thoracic aortic guidelines recommend that aortic diameter determinations be performed perpendicular to the axis of blood flow (Class I Recommendation; Level C Evidence [ Fig. 19-8]). 6 Furthermore, echocardiographic determination of aortic diameter should measure the internal diameter (intima to intima) perpendicular to the direction of blood flow (Class I Recommendation; Level C Evidence [see Fig. 19-8]). 6 At the aortic root level, the echocardiographic determination should use the widest diameter, which is typically at the mid-sinus level (Class I Recommendation; Level C Evidence). 6 Since aortic diameters measured by computed tomography (CT) are determined from adventitia to adventitia perpendicular to the axis of blood flow, aortic diameters measured by echocardiography are typically slightly smaller (see Fig. 19-8).
TABLE 19-1
Normal Adult Thoracic Aortic Diameters
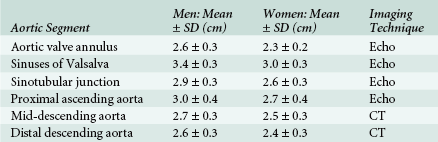
CT, Computed tomography; Echo, echocardiography; SD, standard deviation.
Data from Johnston KW, Rutherford RB, Tilson MD, et al. Suggested standards for reporting on arterial aneurysms. Subcommittee on Reporting Standards for Arterial Aneurysms, Ad Hoc Committee on Reporting Standards, Society for Vascular Surgery and North American Chapter, International Society for Cardiovascular Surgery. J Vasc Surg. 1991;13:452-458; and Roman MJ, Devereux RB, Kramer-Fox R, et al. Two-dimensional echocardiographic aortic root dimensions in normal children and adults. Am J Cardiol. 1989;64:507-512.
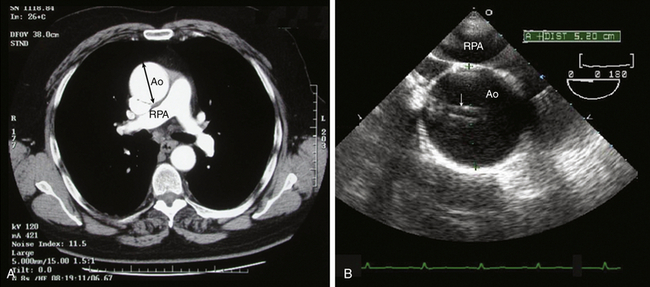
Figure 19-8 Ascending aortic aneurysm with imaging artifacts. A, Computed tomographic angiogram (CTA) of axial slice through ascending aorta (Ao) at level of right pulmonary artery (RPA). B, TEE midesophageal ascending aorta short-axis image at level of RPA. Ascending aorta is dilated. Ascending aortic diameter measured from adventitia to adventitia was 5.4 cm by CTA. Ascending aortic diameter measured from intima to intima was 5.2 cm by TEE. Note that TEE image is complicated by linear imaging artifacts (arrow) as a consequence of reverberation and side lobe artifacts. Linear imaging artifacts are common during TEE examination in patients with a dilated ascending aorta and can be distinguished from the intimal flap of an aortic dissection because they course outside boundaries of ascending aorta. (See Video 19-10 and 19-11. ![]() ) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Definitions of Aortic Enlargement
According to recent guidelines, the spectrum of aortic enlargement is clearly defined. 6 An aortic aneurysm (or true aneurysm) is defined as a focal permanent aortic dilation with a diameter greater than 50% the normal diameter for that aortic segment. 6 Although a true aneurysm has all three aortic wall layers, the intima and media may be so attenuated in giant aneurysms that they are undetectable. An aortic pseudoaneurysm (or false aneurysm) results from aortic rupture, and its wall consists of periarterial connective tissue rather than all the aortic wall layers. 6 If a pseudoaneurysm freely communicates with the intravascular space, it is also termed a pulsating hematoma. Aortic ectasia is defined as aortic dilation less than 150% of the expected diameter for that aortic segment. 6 Aortomegaly is defined as aneurysmal involvement of two or more aortic segments. 6
Thoracic Aortic Imaging with TEE
A Systematic Stepwise Approach ( Box 19-1)
Second, image the aorta in the ME long-axis aortic valve view ( Fig. 19-9; also see Fig. 19-1, A). Measure the aortic root diameters from intima to intima (see Fig. 19-1, B). Analyze aortic valve cusp mobility, aortic root calcification, and the extent of sinotubular junction disease. Interrogate the aortic valve with color Doppler imaging to assess for presence and severity of aortic regurgitation.
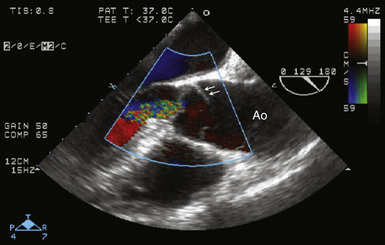
Figure 19-9 Stanford type A dissection shown in midesophageal aortic valve long-axis image at a multiplane angle of 129 degrees. An intimal flap (arrows) is present in aorta (Ao), extending to base of aortic valve cusps within aortic root. Color flow Doppler imaging reveals moderate aortic regurgitation. (See Video 19-3. ![]() ) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Third, image the proximal ascending aorta in short axis at the level of the right pulmonary artery by withdrawing and anteflexing the TEE probe from the aortic valve level (see Fig. 19-2, A). Determine the diameter of the ascending aorta at this level. Examine the aortic wall for signs of atheroma, calcification, and dissection. Remember that the distal ascending aorta and the proximal aortic arch lie in the blind spot of TEE.1,2 Image the proximal ascending aorta in long axis at this level by multiplane rotation to about 90 degrees (see Fig. 19-2, B). Measure the ascending aortic diameter, and assess the aortic wall for signs of atheroma, calcification, and dissection.
Fourth, image the descending thoracic aorta in short axis (see Fig. 19-4, A). Determine the aortic diameter and assess for atherosclerosis, aneurysm, dissection, and pleural effusion (see Fig. 19-4, A). Image the descending thoracic aorta in long axis at this level, with multiplane rotation to approximately 90 degrees (see Fig. 19-4, B). This view facilitates close examination of the aortic intima for signs of disease.
Fifth, interrogate the distal descending thoracic aorta to the diaphragmatic level as the TEE probe is gradually advanced and rotated counterclockwise. The proximal descending thoracic aorta can be interrogated as the TEE probe is gradually withdrawn and rotated clockwise. In this fashion, the entire extent of this aortic segment can be examined, noting that the distal aortic arch is typically 20 to 25 cm from the incisors, the mid-descending thoracic aorta at 30 to 35 cm from the incisors, and the diaphragm at 40 to 45 cm from the incisors. As the aorta is imaged distally, its pulsatility and diameter decrease, especially in the setting of aortic valve disease.24,25 The TEE descending aortic short-axis views also permit detection of left pleural effusions that layer adjacent to the aorta in a supine patient.
Sixth, examine the distal aortic arch as the TEE probe is withdrawn from imaging the distal descending thoracic aorta, noting the distances from the incisors. A long-axis view of the distal aortic arch is typically depicted at a 0-degree multiplane angle (see Fig. 19-3, A). A short-axis view of the distal aortic arch will be displayed by multiplane rotation to 90 degrees (see Fig. 19-3, B). The origins of the brachiocephalic vessels (most often the left subclavian artery) can be sought by rotating the TEE probe clockwise from left to right as the aortic arch is imaged. Color flow Doppler imaging can frequently image blood flow in the brachiocephalic vessels by decreasing the Nyquist limit as needed, since flow is often laminar and off-axis to the ultrasound beam. These views allow detailed assessment of the distal aortic arch with respect to diameter, aneurysm formation, dissection, and atheroma. The upper esophageal aortic arch short-axis view may also display the long-axis view of the pulmonic valve and main pulmonary artery as well as the innominate vein in short-axis ( Fig. 19-10).
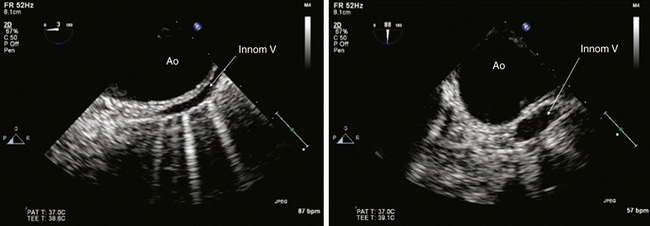
Figure 19-10 Innominate vein shown in upper esophageal aortic arch long-axis view at a multiplane angle of 0 degrees on left and aortic arch short-axis view at a multiplane angle of 88 degrees on right. From these views, it is clear that distal aortic arch (Ao) and innominate vein (Innom V) are intimately related. In long-axis view, anterior aortic wall against adjacent innominate vein mimics an intimal flap of aortic dissection. Short-axis view, however, resolves this artifact by clearly distinguishing the vessels. Imaging a structure of interest in multiple planes minimizes this kind of misinterpretation. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Imaging Artifacts During Echocardiographic Aortic Imaging
Imaging artifacts during echocardiographic evaluation are common, especially in the lumen of a dilated ascending aorta. Since motion artifacts are possible during CT aortic imaging, it is possible they, together with ultrasound imaging artifacts, may be misdiagnosed as the intimal flap of an acute aortic dissection. Artifacts can readily be recognized because they often cross anatomic boundaries and are often not visible in multiple imaging planes.
Linear artifacts result from reverberations between chamber or vessel walls. These reverberations between the walls of the left atrium and/or right pulmonary artery may create a linear artifact in the ascending aortic lumen, resembling an intimal flap of aortic dissection. Note that in Figure 19-8 the linear artifacts course outside the aortic boundaries. Linear artifacts of this type also result from reverberations between a vessel/chamber surface and an intravascular catheter, as in the example of a Swan-Ganz catheter in the pulmonary artery.
Mirroring artifacts are generated when ultrasound is reflected by acoustic interfaces such as the aortic wall. This process results in a mirror image of the aorta adjacent to the interface of interest. A mirror-image artifact juxtaposed to the descending thoracic aorta can mimic aortic dissection. See Chapter 6 for a more detailed explanation of imaging artifacts.
Imaging Pitfalls During Echocardiographic Aortic Imaging
Vessels adjacent to the aortic wall can mimic the appearance of an aortic dissection. The innominate vein appears adjacent to the aortic arch in upper esophageal views and may mimic aortic dissection during long-axis imaging (see Fig. 19-10). The innominate vein can be readily identified by imaging in multiple views and by imaging luminal echocontrast injected into a peripheral vein of the left upper extremity.
Para-aortic effusions may also mimic aortic dissection. A left pleural effusion adjacent to the descending thoracic aorta is readily distinguished from aortic dissection during ME short-axis imaging to depict the characteristic crescent-shaped effusion in the left pleural cavity ( Fig. 19-11). A right pleural effusion can be imaged by rotating the TEE probe to the right, looking for a crescent-shaped fluid collection oriented to the right.
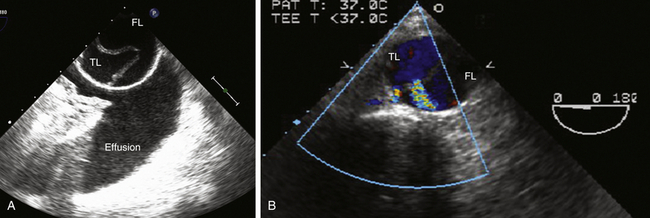
Figure 19-11 A, Dissection of descending thoracic aorta shown in midesophageal (ME) descending aorta short-axis view at a multiplane angle of 0 degrees. There is an aortic dissection, with an intimal flap separating true lumen (TL) from false lumen (FL) of aorta. Pleural effusion in left pleural cavity (effusion) may result from rupture of descending aorta or heart failure. B, ME descending aorta short-axis view at a multiplane angle of 0 degrees showing dissection of descending thoracic aorta. Aortic dissection is evident, with an intimal flap separating true lumen (TL) from false lumen (FL). Doppler color flow interrogation has demonstrated two fenestrations in intimal flap consistent with intimal tears that allow blood flow from true lumen into false lumen. (See Video 19-4 and 19-5. ![]() ) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
) (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Epiaortic Imaging of the Aorta
The aortic root, ascending aorta, and aortic arch can be imaged directly with a 5- to 7-MHz ultrasound transducer in sterile sheath against the anterior aortic wall ( Fig. 19-12).4,5 Epiaortic imaging during cardiac operations is typically feasible after sternotomy, with a systematic comprehensive examination as the standard.3–5 To improve the image quality of near-field structures, an acoustic standoff is typically utilized by filling the mediastinum with warm sterile saline and/or filling the sterile sheath with fluid or ultrasound gel.
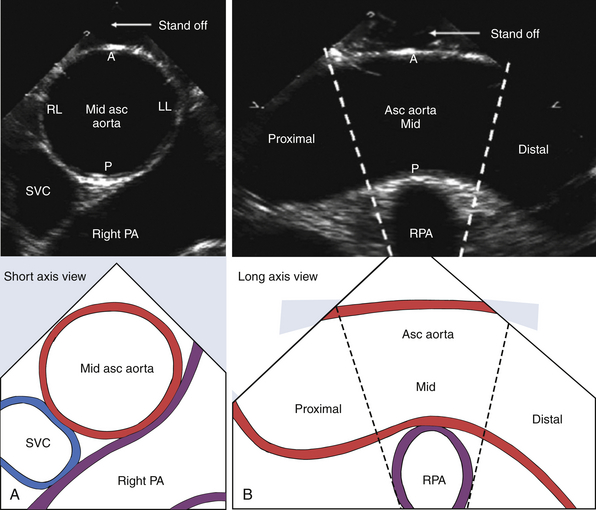
Figure 19-12 Epiaortic imaging of ascending aorta (Asc Aorta) in short axis (A) and long axis (B). Segments of ascending aorta are designated as proximal, mid-, and distal in relation to right pulmonary artery (RPA). Walls of ascending aorta are designated as anterior (A), posterior (P), right lateral (RL), and left lateral (LL). PA, Pulmonary artery; SVC, superior vena cava. (Adapted from Glas KE, Swaminathan M, Reeves ST, et al. Guidelines for the performance of a comprehensive intraoperative epiaortic ultrasonographic examination: recommendations of the American Society of Echocardiography and the Society of Cardiovascular Anesthesiologists; endorsed by the Society of Thoracic Surgeons. J Am Soc Echocardiogr. 2007;20:1227-1235.)
An important indication for epiaortic ultrasound is for assessment of ascending aortic atheroma or calcification prior to manipulation and instrumentation of the ascending aorta during cardiac surgery. Typical ascending aortic events that are associated with cerebral atheroembolism include cannulation for cardiopulmonary bypass and aortic clamping. Risk factors for significant ascending aortic atheroma include prior embolic stroke, severe peripheral vascular disease, severe aortic stenosis, aortic atheroma on preoperative radiographic imaging, and severe atherosclerosis of the descending thoracic aorta detected by TEE.26,27 Epiaortic imaging can also assess for aneurysm and dissection in the ascending aorta and aortic arch where TEE imaging is inadequate, such as in the TEE blind spot and in patients with contraindications to TEE.1–5
According to recent guidelines, epiaortic imaging planes are oriented as short axis or long axis in relation to the direction of blood flow along the length of the vessel. 5 The aortic walls are designated as anterior, right lateral, left lateral, or posterior (see Fig. 19-12). 5 The proximal ascending aorta is imaged in short axis at a level proximal to the right pulmonary artery. The mid-ascending aorta short axis is imaged at the level of the right pulmonary artery. The distal ascending aorta short axis is imaged at a level distal to the right pulmonary artery and proximal to the aortic arch. The ascending aorta long axis is imaged at the level of the right pulmonary artery, showing portions of the proximal, mid-, and distal ascending aorta. The aortic arch long axis is imaged at the level of the innominate, left carotid, and left subclavian arteries.
Aortic Dissection
Aortic dissection is characterized by an intimal disruption with blood entering the medial layer and stripping the intima from the adventitia. 28 If clinical presentation is within 2 weeks of symptom onset, the dissection is classified as acute. 28 If clinical presentation is over 2 weeks from symptom onset, the dissection is classified as chronic. 29
The diagnostic echocardiographic feature of aortic dissection is an intimal flap within the aortic lumen separating the true lumen of the vessel from the false lumen (see Figs. 19-1, B, 19-9, and 19-11). Color Doppler interrogation may demonstrate intimal tears by demonstrating blood flow across the intimal flap (see Fig. 19-11). Intramural hematoma (IMH) is a variant of aortic dissection characterized by separation of the intima from the adventitia, with a hematoma within the medial layer ( Fig. 19-13). 30 A typical feature of IMH is that echocardiography cannot identify an intimal tear, although at times the hematoma will contain echolucent areas consistent with non-coagulated blood within the media.
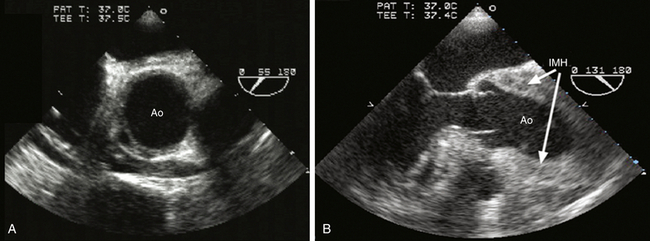
Figure 19-13 Intramural hematoma (IMH) shown in midesophageal views of aortic valve in short axis at a multiplane angle of 55 degrees (A) and in long axis at a multiplane angle of 131 degrees (B). A demonstrates IMH as a variant of aortic dissection as a circumferential or crescent-shaped thickening of aortic wall. In acute IMH, blood may appear within aortic wall as lucent cavities. B depicts longitudinal thickening of wall of the aortic root and ascending aorta caused by intramural hematoma. Ao, Aorta. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
The diagnostic accuracy of TEE in suspected aortic dissection is similar to helical CT and magnetic resonance imaging (MRI), with sensitivity and specificity better than 90%.6,28–31 The clinical portability and diagnostic accuracy of TEE explain its popularity for rapid bedside evaluation of hemodynamically unstable patients with suspected acute aortic dissection ( Fig. 19-14). Recent thoracic aortic disease guidelines recommend urgent and definitive aortic imaging with TEE, CT, or MRI to evaluate for aortic dissection in patients at high risk (Class I Recommendation; Level of Evidence B). 6 In surgical patients in whom the diagnosis of aortic dissection has already been confirmed, comprehensive intraoperative TEE evaluation confirms dissection extent, detects complications of aortic dissection, and guides surgical management ( Fig. 19-15).
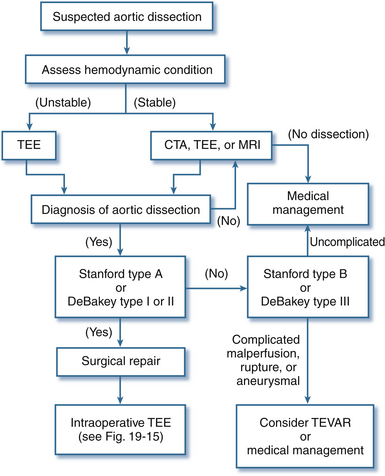
Figure 19-14 Role of transesophageal echocardiography (TEE) for evaluation and diagnosis of aortic dissection. CTA, Computed tomographic angiogram; MRI, magnetic resonance imaging; TEVAR, thoracic endovascular aortic repair. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
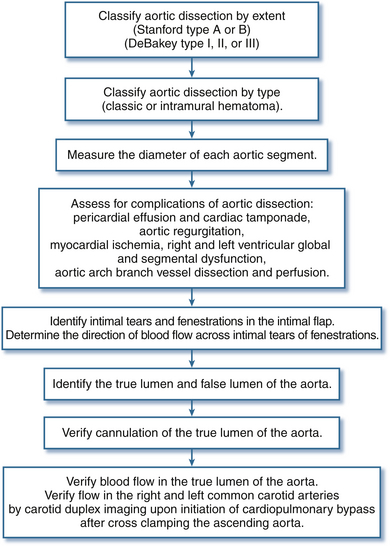
Figure 19-15 Intraoperative transesophageal echocardiography examination for aortic dissection. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
Classification of Aortic Dissection
Aortic dissection can be classified according to intimal tear site and dissection extent ( Fig. 19-16). The DeBakey scheme has three main subtypes. The Stanford scheme has two main subtypes. The classification of aortic dissection is clinically important for clinical management, including prognosis. Acute aortic dissections that involve the ascending aorta are typically surgical emergencies, since delays in surgical management are associated with high mortality risk.32,33
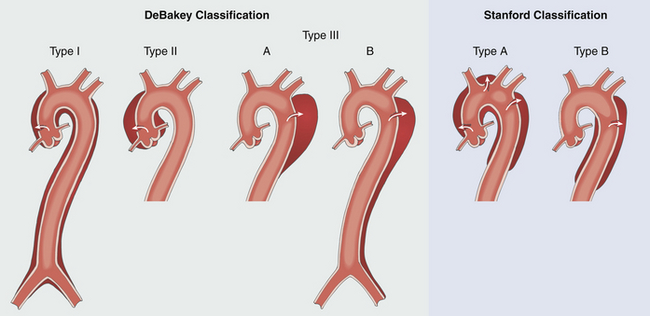
Figure 19-16 Classification of aortic dissection. Two anatomic classifications of aortic dissection focus on intimal tear site and extent of dissection: DeBakey scheme has three main subtypes, and Stanford scheme has two main subtypes. Acute aortic dissections involving ascending aorta are typically considered surgical emergencies (DeBakey I and II; Stanford type A). (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
The Stanford classification emphasizes the clinical importance of ascending aortic dissection (see Fig. 19-16). Stanford type A aortic dissection is defined as any dissection involving the ascending aorta, regardless of intimal tear site (surgical repair typically recommended). Stanford type B dissection is defined as any dissection that does not involve the ascending aorta (medical management recommended unless complicated by threatened/frank aortic rupture and/or malperfusion).28,29
The DeBakey classification focuses on intimal tear site and dissection extent (see Fig. 19-16). DeBakey type I dissection is characterized by an intimal tear within the ascending aorta complicated by dissection involving the ascending aorta, aortic arch, and descending aorta (surgical repair recommended). DeBakey type II dissection is characterized by an intimal tear within the ascending aorta complicated by dissection confined to the ascending aorta (surgical repair recommended). DeBakey type III dissection is characterized by an intimal tear within the descending thoracic aorta complicated by dissection of the descending aorta (medical management recommended unless complicated by threatened/frank aortic rupture and/or malperfusion). If dissection in this setting is confined to the descending thoracic aorta, it is defined as DeBakey type IIIa. If dissection in this setting involves the descending aorta and abdominal aorta, it is DeBakey type IIIb.
Although ascending aortic dissection is a surgical emergency, perioperative mortality depends significantly on clinical presentation, specifically the presence of branch vessel malperfusion (regional ischemia) and/or circulatory compromise (generalized ischemia).34,35 Recently, the Penn classification of clinical presentation in acute type A dissection has been derived and validated to focus future interventions on clinical presentations in this life-threatening disease as part of the collective effort to improve clinical outcomes yet further.34,35 Penn Class A presentations are characterized by the absence of ischemia, specifically the absence of malperfusion and circulatory compromise. Penn Class B presentations are characterized by branch vessel malperfusion (e.g., stroke, cold pulseless upper extremity). Penn Class C presentations are characterized by circulatory compromise including shock and/or cardiac arrest. Penn Class B&C presentations are characterized by both branch vessel and circulatory compromise. The clinical presentation in acute type A dissection as defined by the Penn classification independently predicts perioperative mortality.34,35 An integrated Penn classification of Stanford acute type A aortic dissection has recently been proposed to encourage a comprehensive management strategy of this acute aortic syndrome by considering Penn clinical presentation and DeBakey extent of dissection. 36 This integration of the three classifications of ascending aortic dissection will likely frame future advances in diagnosis and management of this surgical emergency. 36
The European classification of acute aortic dissection consists of five classes and focuses on the anatomic spectrum of aortic dissection, including its variants ( Fig. 19-17). 37 Class I is defined as classic aortic dissection with an intimal tear, complicated by an intimal flap separating the true and false aortic lumens. Class II is defined as IMH with hematoma formation within the medial layer. An intimal tear is not usually detected by echocardiography. Class III is defined as limited dissection confined to a short aortic segment. A partial tear of the inner aortic wall is termed a subtle dissection, and scar formation at this partial tear site is termed an abortive discrete dissection. Class IV is defined as a penetrating atherosclerotic ulcer (PAU) with localized hematoma or pseudoaneurysm. Class V is defined as traumatic or iatrogenic aortic dissection, typically associated with aortic catheterization or surgical aortic manipulation.
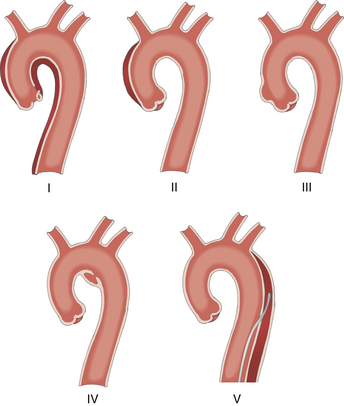
Figure 19-17 European classification for variants of aortic dissection: class I, classic aortic dissection; class II, intramural hematoma; class III, subtle discrete aortic dissection; class IV, plaque rupture or ulceration; class V, traumatic or iatrogenic aortic dissection. (Adapted from Cheung AT, Weiss SJ. Diseases of the aorta. In: Oxorn DC, ed. Intraoperative Echocardiography. Philadelphia: Saunders; 2012:161-182.)
As a variant of aortic dissection, IMH is characterized by a medial hematoma that may also be subadventitial. 38 It accounts for up to 30% of patients presenting with an acute aortic syndrome (see Fig. 19-13), especially in Asia.39,40 The typical patient with IMH is elderly, hypertensive, and has established peripheral vascular disease.38,39 The genesis of IMH is still debated as to whether it is aortic dissection with a thrombosed false lumen or whether it is intramedial hemorrhage from rupture of the vasa vasorum.38,41 The extent of IMH is classified by the Stanford approach as type A and type B. Indications for surgery in type A IMH include refractory pain, ascending aortic diameter 5 cm or greater, hematoma thickness greater than 1 cm, and hemodynamic instability. 38
As a variant of aortic dissection, PAU is a focal atherosclerotic plaque that corrodes through the internal elastic lamina into the media.38,39
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


