Hypertrophic cardiomyopathy (HC) is the most common cause of sudden death in the young, but survival to particularly advanced age is less well appreciated. The investigators report the prevalence, clinical features, and demographics of patients with HC surviving to ≥90 years of age. Of 1,297 patients with HC in the Hypertrophic Cardiomyopathy Center database (Minneapolis Heart Institute Foundation), 26 (2.0%) were identified who had achieved the age of ≥90 years; 18 (69%) were women. HC diagnosis came late in life, at 61 to 92 years (mean 80 ± 8; ≥75 years in 21 patients), recognized fortuitously by the detection of a heart murmur or during family screening (n = 6) or after onset of new symptoms (n = 20). At most recent evaluation (or death) patients were aged 90 to 96.7 years (mean 92.2 ± 2), with 6 presently alive at 91 to 96 years of age; HC did not appear to be the primary cause of death in any patient. Left ventricular wall thicknesses were 15 to 31 mm (mean 20 ± 3); 8 patients (31%) had obstruction to left ventricular outflow at rest (peak instantaneous gradients, 38 to 135 mm Hg). Significant HC-related complications occurred in 13 patients (50%), including progressive heart failure symptoms, atrial fibrillation, and nonfatal embolic stroke. Although no patient died suddenly, 13 (50%) nevertheless carried conventional HC risk markers. A greater proportion of cohort patients reached ≥90 years of age (2.0%) than expected in the general population (0.8%) (p <0.001). In conclusion, HC may be unrecognized until late in life and is consistent with survival to particularly advanced age into the 10th decade of life without the need for major HC-related treatment interventions, and with demise ultimately largely unrelated to this disease. This principle regarding the natural history of HC can afford a measure of reassurance to many patients.
The risks and disease complications associated with hypertrophic cardiomyopathy (HC) have historically been emphasized for young patients. However, it remains less well appreciated that HC is compatible with normal life expectancy and especially with survival to particularly advanced ages. Notably, in this regard, we have identified a subgroup of patients among our large HC cohort, characterized by the most extended survival for ≥90 years. We report the demographics and clinical courses of this patient subset with the expectation that these data will importantly extend our understanding of the natural history of HC.
Methods
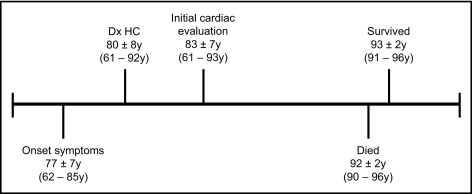
The database of the Hypertrophic Cardiomyopathy Center of the Minneapolis Heart Institute Foundation was accessed. Of the 1,297 patients, 254 (20%) have survived to age 70 years and 107 (8%) to age 80 years, and 26 (2%) were identified to have achieved ≥90 years of age and constituted the present study group ( Figure 1 ) . The time elapsed from the initial clinical visit at our center to the most recent evaluation (or death) on March 1, 2011, constituted a follow-up period of 9.4 ± 7 years.
HC was defined as echocardiographic demonstration of a hypertrophied and nondilated left ventricle in the absence of another cardiac or systemic disease capable of producing the degree of wall thickening evident. Specifically, 6 study patients had histories of pharmacologically controlled and mild systolic hypertension, judged insufficient in magnitude and duration to account for the degree of left ventricular (LV) hypertrophy observed (≥19 mm). In addition, LV outflow tract obstruction at rest was present in 1 of these patients, inconsistent with systemic hypertension, and strong family histories of HC were evident in 2 others.
Echocardiographic studies were performed with commercially available instruments. The magnitude and distribution of LV hypertrophy were assessed in all cross-sectional planes. Maximum LV wall thickness was defined as the greatest dimension measured at any site within the LV chamber. Left atrial and LV end-diastolic cavity dimensions were assessed using M-mode echocardiography in a standard fashion. Peak instantaneous outflow gradient was estimated under basal conditions with continuous-wave Doppler echocardiography.
In 2 patients, cardiovascular magnetic resonance imaging studies were performed with a 1.5-T clinical scanner (Sonata; Siemens Healthcare, Erlangen, Germany). Late gadolinium enhancement images were acquired 10 to 15 minutes after the intravenous administration of 0.2 mmol/kg gadolinium diethylenetriamine penta-acetic acid (Magnevist; Schering AG, Berlin, Germany) with a breadth-held segmented inversion recovery sequence, acquired in the same orientations as the cine images.
For patients with HC, the fraction surviving at each follow-up time was estimated using the Kaplan-Meier method. The expected fraction surviving at each time after initial evaluation was computed by assigning to each patient the probability of surviving after presentation, appropriate to patient age and gender on the basis of known mortality rates from Minnesota and the general United States population. Actual and expected surviving fractions were compared using the 1-sample log-rank test, which also provides an estimate and confidence interval for the standardized mortality ratio and 95% confidence interval. All computations used are from the survival package version 2.36-S of the R software system version 2.13.O (R Development Core Team, Vienna, Austria).
Descriptive statistics are expressed as mean ± SD for continuous variables and as proportions for categorical variables. Continuous variables were assessed using paired or unpaired Student’s t tests as appropriate, and categorical measures were compared using standard chi-square tests. Statistical analyses were performed using Stata version 11.1 (StataCorp LP, College Station, Texas), and statistical significance was defined as p <0.05.
Results
The 26 patients ranged in age from 61 to 93 years (mean 83 ± 7) at initial cardiac evaluation at our institution and from 90 to 96.7 years (mean 92 ± 2) at last follow-up (or death) ( Table 1 , Figure 1 ), including 2 patients born in 1893 and 1901 and 6 others before 1910. Eighteen patients (69%) were women; 25 were white, and 1 was black.
| Patient | Gender | Age at Diagnosis | Age I | Age II (Death) | NYHA Class I | NYHA Class II | Age at Onset of Exertional Symptoms | Echocardiography | CAD | SH | AF | Cause of Death | Drugs (Initial Evaluation) | Potential HC Risk Factors | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maximum LVWT (mm) | LVED (mm) | LA (mm) | EF (%) | LVOTG (mm Hg) | ||||||||||||||
| 1 | F | 84.0 | 91.1 | (91.3) | 2 | 2 | 81 | 20 | 54 | 42 | 50 | 0 | — | + | 0 | HF: multiple-organ failure | BB | 0 |
| 2 | M | 73.2 | 74.5 | (91.2) | 3 | 3 | 72 | 20 | 42 | 58 | 73 | 0 | + | + | 0 | Cancer | BB; CA; isosorbide dinitrate | 0 |
| 3 | F | 83.5 | 83.5 | (95.0) | 3 | 3 | 79 | 19 | 34 | 47 | — | 0 | — | 0 | C | UNK (NH) | BB; CA; quinidine | 0 |
| 4 | F | 91.3 | 91.3 | (91.8) | 1 | 1 | NA | 20 | 32 | 40 | 65 | 135 | — | 0 | C | UNK (NH) | BB; CA; diuretic; digitalis | FHSD-HC |
| 5 | F | 87.4 | 87.4 | (90.0) | 2 | 2 | 83 | 18 | 40 | 50 | 70 | 20 | — | 0 | P † | HF: multiple-organ failure | BB; paroxetine; warfarin | 0 |
| 6 | F | 64.8 | 78.0 | (92.8) | 2 | 2 | 64 | 18 | 35 | 40 | — | 0 | + | 0 | P † | HF: CAD (MI) | ACEI/ARB; warfarin; diuretic; digoxin | Syncope |
| 7 | F | 82.8 | 89.4 | (91.3) | 1 | 1 | NA | 18 | 37 | 38 | 80 | 0 | — | 0 | 0 | UNK (NH) | BB | Syncope |
| 8 | F | 79.9 | 79.9 | (90.5) | 2 | 2 | 78 | 19 | 44 | 42 | 60 | 65 | — | + | 0 | Dementia | BB | 0 |
| 9 | F | 83.9 | 83.9 | (92.9) | 2 | 2 | 84 | 21 | 41 | 35 | 65 | 0 | — | 0 | 0 | UNK (NH) | BB | NSVT ⁎ |
| 10 | F | 62.2 | 62.2 | (90.0) | 1 | 2 | 62 | 20 | 32 | 39 | 70 | 0 | — | 0 | 0 | Aortic aneurysm ruptured | BB | Syncope |
| 11 | F | 77.7 | 77.7 | 94.0 | 1 | 1 | NA | 15 | 45 | 34 | 65 | 0 | — | + | 0 | NA | 0 | FHSD-HC |
| 12 | F | 78.3 | 78.3 | (91.3) | 1 | 1 | NA | 18 | 32 | 36 | 65 | 60 | — | 0 | 0 | UNK (NH) | 0 | 0 |
| 13 | F | 73.9 | 74.8 | 96.0 | 1 | 1 | NA | 23 | 42 | 33 | 70 | 0 | — | + | 0 | NA | BB; diuretic | 0 |
| 14 | M | 61.0 | 78.3 | (90.0) | 1 | 1 | NA | 28 | 54 | 38 | 60 | 0 | — | 0 | C | UNK | Warfarin; diuretic; quinidine | Syncope |
| 15 | M | 79.2 | 91.0 | 91.4 | 1 | 1 | NA | 19 | 43 | 40 | 65 | 75 | — | 0 | 0 | NA | BB | 0 |
| 16 | M | 75.4 | 84.8 | (92.1) | 2 | 1 | 73 | 31 | 23 | 52 | 80 | 23 | + | 0 | P | Multiple-organ failure; CAD with CABG | BB; CA | NSVT ⁎ ; massive LVH |
| 17 | F | 83.0 | 83.6 | (91.5) | 1 | 1 | NA | 18 | 50 | 50 | 70 | 65 | — | 0 | P | UNK (NH) | CA | Syncope |
| 18 | F | 80.3 | 80.3 | (90.5) | 3 | 3 | 80 | 18 | 40 | 48 | 60 | 100 | — | 0 | P | HF: multiple-organ failure | BB; CA; disopyramide | 0 |
| 19 | F | 77.7 | 78.3 | 94.6 | 1 | 1 | NA | 15 | 31 | 40 | 65 | 0 | — | 0 | P | NA | BB; ASA | 0 |
| 20 | F | 76.9 | 76.9 | (92.4) | 3 | 2 | 74 | 20 | 40 | 40 | 65 | 0 | + | 0 | 0 | HF: CAD; stent; ICD § | BB; diuretic; digitalis | Syncope; NSVT ⁎ |
| 21 | F | 83.9 | 83.9 | (95.6) | 3 | 3 | 81 | 19 | 36 | 37 | 65 | 0 | — | 0 | 0 | Cancer | 0 | 0 |
| 22 | F | 84.9 | 84.9 | (96.7) | 2 | 2 | 85 | 21 | 31 | 32 | 75 | 64 | — | 0 | 0 | UNK (NH) | BB; CA; diuretic | 0 |
| 23 | M | 86.3 | 86.3 | (90.4) | 1 | 1 | NA | 22 | 54 | 51 | 65 | 0 | — | + | 0 | GI bleed | ACEI/ARB; CA; diuretic | FHSD-HC |
| 24 | M | 90.4 | 90.4 | 90.7 | 1 | 1 | NA | 20 | 49 | 46 | 60 | 0 | — | 0 | 0 | NA | ACEI/ARB | NSVT ⁎ |
| 25 | M | 87.0 | 89.8 | (91.2) | 3 | 3 | 80 | 22 | 43 | 48 | 63 | 38 | — | 0 | 0 | Hemorrhagic stroke | CA | 0 |
| 26 | M | 92.0 | 93.3 | 93.3 | 1 | 1 | NA | 18 ‡ | 52 | 36 | 60 | 0 | + | 0 | 0 | NA | Diuretic | NSVT |
⁎ NSVT identified on 24-hour ambulatory (Holter) electrocardiographic monitoring.
† Associated with stroke, judged to be embolic.
§ Secondary prevention ICD intervened appropriately at age 90 years.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


