A calcified nodule is a type of potentially vulnerable plaque accounting for approximately 2% to 7% of coronary events. Because its intravascular ultrasound (IVUS) features have never been validated, the aim of this study was to assess the IVUS characteristics of calcified nodules in comparison to histopathology. IVUS was performed in 856 pathologic slices in 29 coronary arteries (11 left anterior descending, 5 left circumflex, and 13 right coronary arteries) in 18 autopsy hearts. Pathologic sections were analyzed every 2 mm; qualitative and quantitative findings of matched IVUS were analyzed. IVUS detected calcification in 285 frames; 17 (6.0%) were calcified nodules, and 268 (94.0%) were non-nodular calcium by histopathology. Two calcified nodules (11.8%) were solitary, and 15 (88.2%) were adjacent to non-nodular calcium. IVUS characteristics of calcified nodules were (1) a convex shape of the luminal surface (94.1% in calcified nodules vs 9.7% in non-nodular calcium, p <0.001), (2) a convex shape of the luminal side of calcium (100% vs 16.0%, p <0.001), (3) an irregular luminal surface (64.7% vs 11.6%, p <0.001), and (4) an irregular leading edge of calcium (88.2% vs 19.0%, p <0.001). Luminal area at the calcified nodule site was larger (6.2 ± 2.4 vs 4.3 ± 1.6 mm 2 , p <0.001) and plaque burden less (57 ± 6% vs 68 ± 5%, p <0.001) than at the minimum luminal area site. In conclusion, calcified nodules have distinct IVUS features (irregular and convex luminal surface) permitting their prospective identification in vivo.
Intravascular ultrasound (IVUS) has high sensitivity and specificity for the detection of intracoronary calcium, but the differentiation between calcified nodules and non-nodular calcium has never been validated. To assess the frequency and distribution of calcified nodules in vivo, it is first necessary to validate their detection using intravascular imaging techniques. Therefore, we used coronary arteries from human autopsied hearts to validate the IVUS characteristics of calcified nodules in comparison to histopathology.
Methods
The arteries used in the present analysis have been reported previously. Vessels were obtained over a 2-year period from 84 autopsied human hearts. Among them, there were 7 arteries in 5 patients with ≥1 calcified nodule and a randomly chosen group of 22 arteries in 13 patients without calcified nodules (control group). IVUS was compared to the histopathologic standard. Hearts were acquired from the National Disease Research Interchange or the International Institute for the Advancement of Medicine as per a protocol approved by the institutional review board. Hearts were received <48 hours after death, maintained on ice at 4°C, and imaged <96 hours after death. Each arterial segment was then mounted in a custom fixture, and the 2 ends of the segment were attached to lure connectors that allowed fluid flow and catheter entry. The segment was perfused with pulsatile human blood using a varistaltic pump (Manostat; Barnant Corporation, Barrington, Illinois) at body temperature.
IVUS imaging was performed with an Atlantis SR Pro 40-MHz catheter (Boston Scientific Corporation, Fremont, California) using motorized pullback at 0.5 mm/s to include proximal and distal lure connectors. IVUS studies were archived onto CD-ROMs and sent to an independent IVUS core laboratory (Cardiovascular Research Foundation, New York, New York) for quantitative and qualitative analyses using validated planimetry software (EchoPlaque; INDEC Systems, Inc., Mountain View, California). Every histopathologic slice and its comparable IVUS frame was matched using vessel shape, side branches, perivascular structures, and distances from the lure connectors.
Qualitative analysis included (1) plaque shape at the luminal surface (concave or convex), (2) calcium shape (concave or convex), (3) luminal surface (smooth or irregular, nonsmooth, and lumpy), (4) calcium surface (smooth or irregular, nonsmooth, and lumpy), (5) luminal surface echogenicity (hypoechoic, isoechoic, or hyperechoic), (6) visible plaque between the lumen and calcium (absent or present), (7) calcium location (superficial, mixed, or deep), (8) eccentricity of plaque (concentric, eccentric, or eccentric with arc of normal vessel), and (9) plaque morphology (isoechoic, mixed, or calcified). Quantitative analysis was performed according to published standards including external elastic membrane (EEM), lumen, and plaque and media (calculated as EEM minus lumen) cross-sectional area and plaque burden (plaque and media divided by EEM) at the site of the calcium nodule, minimum luminal cross-sectional area (MLA) site, and the most normal looking reference sites ( Figure 1 ) . The remodeling index was the lesion site divided by the average of the proximal and distal reference EEM cross-sectional area. Positive, intermediate, and negative remodeling were defined as remodeling indexes >1.05, 0.95 to 1.05, and <0.95, respectively.
After the IVUS images were obtained, the segments were pressure-fixed in formalin and decalcified with ethylenediaminetetraacetic acid. Each segment was then cut perpendicular to the longitudinal axis of the vessel at 2-mm intervals. Two 5-μm histopathologic slides were prepared at the CVPath Institute (Gaithersburg, Maryland) from the distal site of each 2-mm block and stained with hematoxylin and eosin and Russell-Movat’s pentachrome. Each histopathologic slide was digitized using a standard microscope at 1.25× magnification. Histologic features of the arteries were classified by a CVPath pathologist (A.P.B.) according to a modified American Heart Association classification scheme.
SPSS for Windows version 13.0 (SPSS, Inc., Chicago, Illinois) was used for all analyses. Continuous variables were compared using Student’s t tests and are presented as mean ± SD. If the normality assumption was violated, nonparametric Wilcoxon’s rank-sum test was used, and variables are presented as medians with first and third quartiles. Categorical variables were compared using chi-square or Fisher’s exact tests as appropriate and are presented as frequencies. A p value <0.05 was considered statistically significant.
Results
A total of 856 blocks from 29 arteries (11 left anterior descending, 5 left circumflex, and 13 right coronary arteries) were used for the present calcified nodule validation study ( Table 1 ). IVUS detected calcification in 285 cross sections; 17 (6.0%) were calcified nodules, and 268 (94.0%) contained non-nodular calcium by histopathology. Two calcified nodules (11.8%) were solitary, and 15 (88.2%) were adjacent to non-nodular calcium. Among the 29 arteries, 7 (2 left anterior descending, 1 left circumflex, and 4 right) contained ≥1 calcified nodule; conversely, 22 arteries (9 left anterior descending, 4 left circumflex, and 9 right) contained no calcified nodules.
| Modified American Heart Association Classification | Number of Sections |
|---|---|
| Adaptive intimal thickening or normal | 276 (32.2%) |
| Smooth muscle cell rich plaque or not otherwise specified | 63 (7.4%) |
| Bland fibrous plaque or not otherwise specified | 62 (7.2%) |
| Intimal xanthoma | 13 (1.5%) |
| Pathologic intimal thickening | 86 (10.0%) |
| Calcified fibrous | 195 (22.8%) |
| Calcified nodule | 17 (2.0%) |
| Early fibroatheroma | 43 (5.0%) |
| Early fibroatheroma with calcified core | 21 (2.5%) |
| Late fibroatheroma | 42 (4.9%) |
| Late fibroatheroma with calcified core | 25 (2.9%) |
| Thin-capped fibroatheroma | 12 (1.4%) |
| Thin-capped fibroatheroma with calcified core | 0 (0%) |
| Ruptured plaques | 1 (0.1%) |
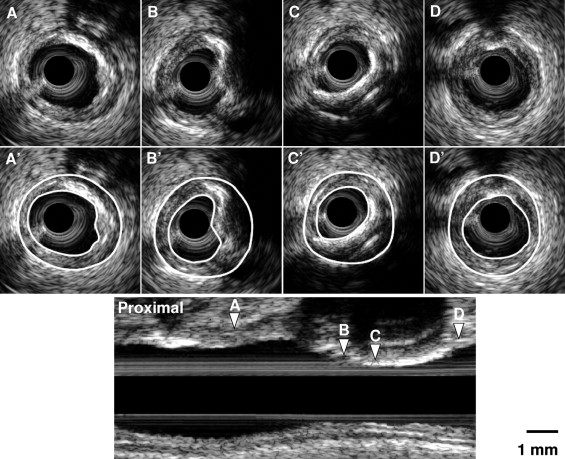
IVUS characteristics of calcified nodules were (1) a convex shape of the luminal surface (present in 94.1% of calcified nodules vs 9.7% of non-nodular calcium, p <0.001); (2) a convex shape of calcium (100% vs 16.0%, p <0.001); (3) an irregular, nonsmooth, and lumpy luminal surface (64.7% vs 11.6%, p <0.001); and (4) an irregular, nonsmooth, and lumpy leading edge of calcium (88.2% vs 19.0%, p <0.001) ( Table 2 ). An example of a calcified nodule is shown in Figure 2 . Table 3 compared the quantitative analysis at the calcified nodule site versus the MLA site of non-nodular calcium. Overall, 64.7% of calcified nodules were located distal to the MLA site (range, 0.2 to 14.0 mm) and the rest proximal to the MLA site (range, 3.0 to 33.0 mm), but none at the corresponding MLA site.
| IVUS Variable | Calcified Nodule (n = 17) | Non-Nodular Calcium (n = 268) | p Value |
|---|---|---|---|
| Plaque shape of luminal surface | |||
| Concave | 1 (5.9%) | 242 (90.3%) | <0.001 |
| Convex | 16 (94.1%) | 26 (9.7%) | |
| Shape of luminal side of calcium | |||
| Concave | 0 (0%) | 225 (84.0%) | <0.001 |
| Convex | 17 (100%) | 43 (16.0%) | |
| Luminal surface irregularity | |||
| Regular | 6 (35.3%) | 237 (88.4%) | <0.001 |
| Irregular | 11 (64.7%) | 31 (11.6%) | |
| Irregularity of calcium | |||
| Regular | 2 (11.8%) | 217 (81.0%) | <0.001 |
| Irregular | 15 (88.2%) | 51 (19.0%) | |
| Luminal surface echogenicity | |||
| Hypoechoic | 1 (5.9%) | 10 (3.7%) | 0.257 |
| Isoechoic | 13 (76.5%) | 158 (59.0%) | |
| Hyperechoic | 3 (17.6%) | 100 (37.3%) | |
| Visible tissue between lumen and calcium | |||
| Absent | 4 (23.5%) | 42 (15.7%) | 0.287 |
| Present | 13 (76.5%) | 226 (84.3%) | |
| Calcium location | |||
| Superficial | 15 (88.1%) | 189 (70.5%) | 0.234 |
| Mixed | 2 (11.8%) | 52 (19.4%) | |
| Deep | 0 (0%) | 27 (10.1%) | |
| Eccentricity of plaque | |||
| Concentric | 1 (5.9%) | 54 (20.1%) | 0.327 |
| Eccentric | 9 (52.9%) | 130 (48.5%) | |
| Eccentric with normal wall | 7 (41.2%) | 84 (31.3%) | |
| Plaque morphology | |||
| Isoechoic | 0 (0) | 4 (1.5%) | 0.024 |
| Mixed | 2 (11.8) | 118 (44%) | |
| Calcified | 15 (88.2%) | 146 (54.5%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


