Hemodynamic Assessment and Transcatheter Therapy for Congenital Heart Disease
Matthew J. Gillespie
Jonathan J. Rome
Advances in noninvasive imaging have done away with the need for preoperative cardiac catheterization in the management of many heart lesions. Thus, cardiac catheterization is not indicated prior to operative intervention for transposition of the great arteries, hypoplastic left heart syndrome (HLHS), truncus arteriosus, complete common atrioventricular canal defect, a simple paramembranous ventricular septal defect, or tetralogy of Fallot, unless particular concerns arise. This discussion of heart catheterization in children, therefore, focuses not on which lesions require a heart catheterization for a complete evaluation but rather what information is desired when it has been decided that a heart catheterization should be undertaken.
BASIC PRINCIPLES OF HEMODYNAMIC EVALUATION
Invasive hemodynamic assessment involves calculation of flows and resistances, shunt calculations, and measurement of pressure and flow gradients.
Calculation of Flows
The simplest method of measuring flow is to use a thermodilution catheter. This method is a variation on the dye dilution method using temperature decrement to determine blood flow. However, the method is only applicable in the absence of intracardiac shunting when there is a mixing chamber between the proximal and distal ports. Since most children with congenital heart disease do have either right-to-left, left-to-right, or bidirectional shunting, thermodilution is not an appropriate method of determining cardiac output in these patients.
More often, the Fick principle is used for calculation of flow. The essence of the Fick principle is that flow can be calculated by the use of a measurable indicator, which is added or subtracted at a known rate. In practice, the indicator is oxygen.
F = unknown flow rate (ml/min)
I1, I2 = indicator concentrations (mg/ml) at the two ends of the flow pathway
R = rate at which indicator is added or subtracted (mg/min)
F (ml/min) = R (mg/min)/I2 − I1 (mg/ml)
In a hemodynamic study, the unknown flow rate is systemic or pulmonary blood flow, the indicator is oxygen, and the rate of change of the indicator is the oxygen consumption. Oxygen content is determined by multiplying the hemoglobin concentration times the spectrophotometrically measured percentage of oxygen saturation of hemoglobin; dissolved oxygen must be included in the calculation when patients receive supplemental oxygen. Oxygen consumption is either measured or assumed on the basis of age and heart rate, using standardized tables. The equation for calculation of pulmonary blood flow is as follows:
QP = O2 consumption/(pulmonary vein O2 content – pulmonary artery O2 content)
and the equation for calculation of systemic blood flow is as follows:
QS = O2 consumption/(aortic O2 content − venous O2 content).
Effective flow describes the quantity of deoxygenated blood that flows through the pulmonary circuit, which is also equal to the volume of oxygenated blood that flows through the systemic circuit. QP effective equals QS effective and is calculated by taking the difference between the mixed systemic and pulmonary venous oxygen content and dividing that into the oxygen consumption:
Qeff = O2 consumption/(pulmonary venous O2 content – mixed venous O2 content)
Calculation of Shunts
Shunt flow can be thought of as the ineffective flow in the system: the left-to-right shunt is the volume of oxygenated blood flowing through the lungs and is equal to the total pulmonary flow minus the effective pulmonary flow (QP – Qeff). The right-to-left shunt is the volume of deoxygenated blood flowing through the systemic circulation and is equal to the total systemic flow minus the effective systemic flow (QS – Qeff).
Because of the great variation in size among pediatric patients, flows are always indexed to body surface area.
Calculation of Resistance
Resistance is the change in mean pressure divided by the flow. Thus, pulmonary vascular resistance (Rp) is the difference between the pulmonary venous and pulmonary arterial pressures divided by the total pulmonary flow.
Rp = (mean pulmonary vein pressure − mean pulmonary arterial pressure)/QP
and the systemic vascular resistance is the difference between the systemic arterial and right arterial pressures divided by systemic flow.
Rs = (mean arterial pressure – mean right atrial pressure)/QS
The resulting units, liters per minute per millimeters of mercury, are termed Wood’s units, after the cardiologist Paul Wood. As with flows, vascular resistance is indexed to body surface area in pediatric patients.
Flow Gradients
In the cardiac catheterization laboratory, gradients across stenotic valves or vessels are generally measured as “peak-to-peak” gradients. This measurement is often inaccurately referred to as the PSEG or peak systolic ejection gradient. The peak-to-peak gradient is a different entity than the maximal instantaneous gradient that is estimated noninvasively by Doppler studies. Whether a gradient is expressed as peak-to-peak, maximal instantaneous gradient, or mean gradient, it is not a
meaningful number unless one has some idea of the amount of flow that is producing the gradient. For example, a patient with critical aortic stenosis who is in low cardiac output may have a relatively low measured gradient across a severely narrowed valve orifice.
meaningful number unless one has some idea of the amount of flow that is producing the gradient. For example, a patient with critical aortic stenosis who is in low cardiac output may have a relatively low measured gradient across a severely narrowed valve orifice.
HEMODYNAMIC EVALUATION OF PATIENTS WITH A LEFT-TO-RIGHT SHUNT LESION
Patients with left-to-right shunt lesions (ventricular septal defect, complete or partial common atrioventricular canal defect, patent ductus arteriosus [PDA]) undergo cardiac catheterization in order to address the following related questions:
What is the magnitude of the left-to-right shunt? Does it justify surgical repair?
What is the status of the pulmonary vascular bed? Is the pulmonary vascular resistance sufficiently low that a complete repair is possible?
HEMODYNAMIC EVALUATION OF PATIENTS WITH A RIGHT-TO-LEFT SHUNT LESION
In patients with right-to-left shunt lesions (critical pulmonary stenosis status post-surgical or transcatheter correction, pulmonary atresia with intact ventricular septum status postaortopulmonary shunt, Ebstein’s anomaly, tetralogy of Fallot with pulmonary atresia status postright ventricular outflow reconstruction with an open ventricular septal defect, single ventricle status postfenestrated Fontan palliation), there is often some uncertainty as to the adequacy of the right heart or pulmonary vascular bed. Specifically, the question is whether one normal cardiac output can pass through the right heart with acceptable systemic venous and right ventricular pressures. If not, the presence of an atrial or ventricular level combination will often permit adequate cardiac output with acceptable hemodynamic values at the expense of oxygenation. Often, an attempt will be made to temporarily occlude the site of right-to-left shunting during the heart catheterization in order to assess the hemodynamic sequelae. Increased heart rate, increased systemic venous pressure, and decreased cardiac output with occlusion of an atrial level communication are all signs of right heart insufficiency.
HEMODYNAMIC EVALUATION OF PATIENTS WITH COMPLETE MIXING LESIONS AND FUNCTIONAL SINGLE VENTRICLE
Currently, the “ideal” surgical palliation for children with functional single ventricle lesions is the modified Fontan operation, with or without an atrial level right-to-left shunt. This type of surgical palliation requires that the pulmonary vascular resistance be low (<4 Wood’s units), as the separation of the circulations is accomplished by using the functional single ventricle to pump systemic blood flow, while pulmonary blood flow occurs passively across a pressure gradient. Early in life (i.e., in the first year), the goal of management is to provide adequate but limited pulmonary blood flow. In cases of functional single ventricle with pulmonic stenosis, no intervention may be needed in the first few months of life. More often, the desired hemodynamics will be achieved via placement of a pulmonary artery band or an aortopulmonary shunt. Typically, this arterial to pulmonary flow is altered to veno pulmonary flow by 6 months of age with a bidirectional cavopulmonary anastomosis (bidirectional Glenn or “hemi-Fontan” procedure). Completion Fontan procedure (total cavopulmonary anastomosis) is usually performed by 3 years of age. Traditionally, cardiac catheterization has been performed prior to the second and third stages of surgical palliation though the need for routine diagnostic catheterization at these times has been called into question. When diagnostic catheterization is performed prior to the Fontan operation, its purpose is to assess whether “Fontan physiology” will be well tolerated in a given patient. The important questions to be evaluated at cardiac catheterization include:
What is the pulmonary vascular resistance? Patients with pulmonary vascular resistance >3 to 4 Wood’s units are much more likely to manifest high central venous pressures and low cardiac output.
What is the pulmonary artery pressure? Elevated pulmonary artery pressure is not necessarily a contraindication to a modified Fontan operation provided that it is measured in the setting of elevated pulmonary blood flow. However, a mean pulmonary artery pressure greater than about 15 mmHg in the setting of normal or diminished pulmonary blood flow bodes poorly for the outcome after conversion to Fontan physiology.
What is the ventricular filling pressure? After a modified Fontan operation, blood flows passively across the pulmonary vascular bed. The central venous pressure will, therefore, be determined by the ventricular filling pressure and the pulmonary vascular resistance. Elevated ventricular filling pressure may be present as a result of chronic volume overload or ventricular hypertrophy, the latter case in particular is associated with poor outcome after Fontan palliation.
HEMODYNAMIC ASSESSMENT OF SEMILUNAR VALVE STENOSIS OR INSUFFICIENCY (WITHOUT SHUNT)
Pulmonary Valve
The severity of pulmonary valve stenosis is graded according to the peak-to-peak gradient across the valve (trivial, <25 mmHg; mild, 25 to 49 mmHg; moderate, 50 to 79 mmHg; severe, >80 mmHg). In general, the presence of a peak-to-peak gradient of ≥40 mmHg is an indication for treatment. Pulmonary insufficiency is difficult to assess quantitatively in the cardiac catheterization laboratory. A ventricularized pulmonary artery tracing—that is, one in which the diastolic pressure approximates that of the right ventricle—indicates severe pulmonic insufficiency.
Aortic Valve
Although cardiologists will generally grade aortic stenosis in adults according to the calculated effective valve area, in the pediatric population indications for intervention are based on catheter-measured peak-to-peak systolic pressure gradients. Because gradients across a stenotic valve will vary depending on flow and heart rate, cardiac output, left ventricular function, and mitral and aortic valve insufficiency all must be evaluated to arrive at an accurate assessment of left ventricular outflow tract obstruction. Left ventricular outflow tract obstruction may be subvalvar, valvar, or supravalvar.
Aortic valve insufficiency is assessed in a semiquantitative manner in the cardiac catheterization laboratory by angiography. A wide arterial pulse pressure not only may represent important aortic valve insufficiency but may also be seen with aggressive afterload reduction.
HEMODYNAMIC ASSESSMENT OF ATRIOVENTRICULAR VALVE STENOSIS OR INSUFFICIENCY
Tricuspid Valve
Isolated tricuspid stenosis is extremely rare; more often, tricuspid stenosis is seen in the context of right heart hypoplasia and pulmonic stenosis or atresia. Tricuspid stenosis cannot be assessed in the presence of pulmonic atresia because there will be little or no flow across the tricuspid valve in these cases. The presence of a patent foramen ovale that allows right-to-left shunting or right ventricular diastolic dysfunction (both of which are common in right heart hypoplasia) makes assessment of the degree of tricuspid stenosis problematic. Complete evaluation requires balloon occlusion of the atrial communication and simultaneous measurement of right atrial and right ventricular pressures; this may not be technically feasible particularly in neonates and small infants (e.g., the atrial communication is too large to be occluded). However, the maneuver may be quite useful in evaluating right heart competency in older children noting any resultant changes in right atrial pressure, cardiac index, and heart rate with balloon occlusion.
Angiographic assessment of tricuspid insufficiency can be difficult because the catheter in the right ventricle of necessity crosses the tricuspid valve; if the catheter stents the valve open or if some of the holes of the catheter are in the right atrium at the time the injection is made, artifactual insufficiency will result. In addition, ectopic beats will often be associated with induced atrioventricular valve insufficiency.
Mitral Valve
Mitral stenosis is generally graded in terms of calculated valve area by cardiologists treating adults and in terms of anatomy and valve gradient by cardiologists treating children. Both the mean valve gradient (arrived at by digitizing the area between the left atrial or pulmonary artery wedge pressure tracing and the left ventricular pressure tracing during the period when the mitral valve [MV] is open) and the gradient between the a-wave and the left ventricular end-diastolic pressure as well as the absolute left atrial pressure are generally considered in assessing mitral stenosis. Because gradients are meaningful only when considered in association with flow, cardiac output must be assessed to appropriately interpret the severity of obstruction. A mean left atrial pressure >25 mmHg is generally associated with moderate-to-severe reactive pulmonary hypertension.
Mitral insufficiency is assessed in a semiquantitative manner by angiography. This is more reliably accomplished than in the case of the tricuspid valve because the catheter with which the picture is taken usually does not cross the MV and the shape of the left ventricle is such that it accommodates a catheter more easily than does the right. However, entanglement of the catheter in the MV apparatus or stimulation of ventricular premature contractions can result in induced mitral insufficiency.
TRANSCATHETER THERAPY FOR CONGENITAL HEART DISEASE
Introduction
Catheter-directed treatments have assumed a major role in the care of patients with congenital cardiovascular defects. These techniques have replaced surgery as the primary mode of therapy in some instances. In many others, optimal treatment combines staged transcatheter and surgical intervention. It is, therefore, essential that the congenital heart surgeon has a working knowledge of interventional cardiology. This section introduces current catheter interventions for patients with congenital cardiovascular defects, high-lighting therapies particularly useful in combination with surgery.
Catheter interventions for congenital heart defects are quite varied, but in simplistic terms can be classified into: (1) procedures to create or enlarge communications and vessels (septostomies, dilations, and stents); (2) procedures to close vessels or defects (device closures, vascular occlusions); and (3) other (thrombectomy, vascular retrievals). Another commonly performed intervention, radiofrequency ablation, is more appropriately dealt with as part of a discussion of cardiac dysrhythmias.
Creation and Enlargement of Atrial Communications
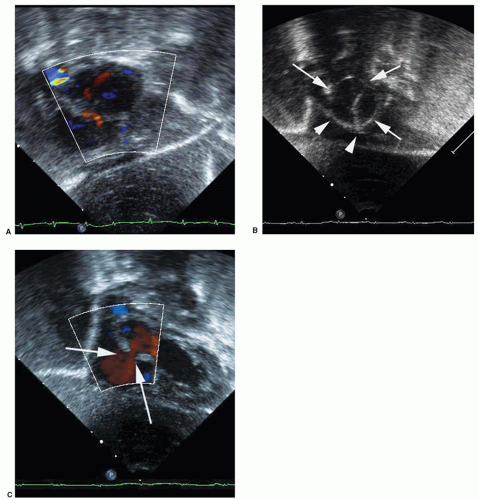
Balloon atrial septostomy described in 1966 by Rashkind and Miller for palliation of cyanosis in D-transposition of the great arteries is often considered the first intervention for congenital heart defects. Even with the use of prostaglandin E1, many infants with transposition require septostomy procedures to ameliorate compromising cyanosis. The procedure is also performed to relieve left atrial hypertension in infants with left atrioventricular valve stenosis or atresia. The basic technique has changed little since first described. A septostomy catheter is advanced through either umbilical or femoral vein into the right atrium and across the foramen ovale. The balloon is filled with fluid and the catheter pulled quickly across the foramen tearing septum primum. Balloon septostomy is often performed at the bedside under echocardiographic guidance (Fig. 74.1).
In many situations, balloon septostomy is not the best way to create an atrial communication. Balloon septostomy is ineffective in patients over 6 weeks of age because the atrial septum is too thick. In most newborns with HLHS and restrictive or intact atrial septum, balloon septostomy is not feasible or ineffective because the left atrium is too small or posterior deviation of septum primum is present. In these populations, an atrial communication is best created by other means. The intact atrial septum may be traversed either by the standard transseptal puncture technique (Brockenbrough procedure), or with the aid of a specially designed radiofrequency perforation system (Nykanen Catheter, Baylis Medical Corp., Mississauga, ON). Once the atrial septum has been crossed, a hole may be created by static balloon angioplasty, or in the case of the very thick atrial septum, endovascular stent deployment. When the procedure is performed for relief of cyanosis in patients with mixing lesions and left atrial outlet obstruction (such as the newborn with HLHS born with intact atrial septum), the goal should be the creation of an unrestrictive atrial communication. In other circumstances (i.e., augmentation of systemic blood flow in patients with right heart failure, or for left atrial decompression in patients on extracorporeal support for left heart dysfunction), smaller atrial communications suffice. In fact, when these techniques are used to palliate right heart failure (transient right ventricular dysfunction after repair of tetralogy of Fallot, or primary pulmonary hypertension, or fenestration creation for Fontan failure), the procedure results in systemic arterial desaturation; thus, the size of communication created must be carefully titrated to arterial oxygen saturation. The goal is to create a restrictive ASD to augment systemic output without excessive cyanosis. In tetralogy of Fallot or pulmonary hypertension patients, this is best accomplished by graded balloon angioplasty of the atrial septum. In Fontan patients requiring fenestration creation, the optimal strategy depends on the type of Fontan. It may include the use of standard or cutting balloon dilation (lateral
tunnel-type Fontan), or transseptal puncture followed by bare metal or covered stent deployment (extracardiac Fontan).
tunnel-type Fontan), or transseptal puncture followed by bare metal or covered stent deployment (extracardiac Fontan).
Valve Repair and Replacement Procedures
Balloon Valvuloplasty
Inflation balloon angioplasty was developed in 1974 by Gruntzig for the treatment of atherosclerotic peripheral arterial stenoses. Advances in equipment and technique have allowed the application of balloon dilation to a variety of valvar and vascular stenoses in patients of all ages.
Pulmonic Valve Stenosis and Atresia
The first congenital lesion treated with balloon dilation was pulmonic stenosis. Balloon valvuloplasty for pulmonic stenosis is usually curative and generally regarded as the treatment of choice in patients of all ages. In older children and adolescents, the procedure is straightforward. After hemodynamic evaluation, a right ventricular angiogram is performed to assess anatomy and measure the pulmonary annulus dimension. Dilation is accomplished by advancing the balloon catheter over a guide wire positioned in the distal pulmonary artery tree through the stenotic valve. The balloon size is chosen to be 120% to 140% of the annulus
diameter. As the balloon is inflated, the stenotic valve creates a waist, which then disappears. Failures may occur (1) if the pulmonary valve is thickened, nondoming, and often muscularized (so-called dysplastic valve common in Noonan’s syndrome) or (2) where valvar stenosis is part of complex obstruction involving infundibulum, valve annulus, or supravalvar region.
diameter. As the balloon is inflated, the stenotic valve creates a waist, which then disappears. Failures may occur (1) if the pulmonary valve is thickened, nondoming, and often muscularized (so-called dysplastic valve common in Noonan’s syndrome) or (2) where valvar stenosis is part of complex obstruction involving infundibulum, valve annulus, or supravalvar region.
Dilation for critical pulmonary stenosis in the newborn is successful in the majority of cases (Fig. 74.2). Moderate cyanosis is common after dilation due to atrial right to left shunting. Cyanosis diminishes as right ventricular compliance improves. Severe cyanosis after valvuloplasty should prompt further evaluation. Where inadequate relief of right ventricular outflow obstruction is present after technically adequate balloon valve dilation, right ventricular outflow patch augmentation may be required, particularly when there is hypoplasia of the pulmonary annulus.
Balloon valvuloplasty is also applicable to newborns with pulmonary atresia and intact ventricular septum. Prior to intervention, these children require diagnostic evaluation to determine that the right ventricle and particularly the infundibulum are of adequate size as well as to rule out the presence of a right ventricular dependent coronary circulation. If an infant is deemed a suitable candidate, the pulmonary valve is perforated with a radiofrequency catheter (see above) and then dilated in the standard manner (Fig. 74.3). Patients undergoing this intervention very frequently develop cyanosis after discontinuation of prostaglandin infusion as the ductus arteriosus closes. If cyanosis occurs, management is individualized. In cases where the right ventricle is deemed well developed, the patient may be maintained on prostaglandins for a time to allow improvement in right ventricular compliance. If the right heart is more significantly hypoplastic, a stable source of additional pulmonary blood flow is added usually by stenting of the ductus arteriosus (Fig. 74.4).
Aortic Stenosis
All treatments for aortic stenosis, including surgical or balloon valvuloplasty, valve replacement, or pulmonary autograft (Ross procedure), should all be considered palliative. Balloon dilation of aortic stenosis generally results in adequate gradient relief with a minimal increase in aortic regurgitation. Reported failure rates vary from 0% to 10%, and significant increases in aortic regurgitation occur in approximately 10% of patients. The duration of successful palliation varies from months to decades though most (if not all) patients will subsequently develop restenosis, progressive regurgitation, or both. Intermediate term results after balloon and surgical valvuloplasty are comparable, thus balloon valvuloplasty is generally recommended as the first treatment for patients with aortic stenosis and little regurgitation. Except in the newborn, aortic valvuloplasty is usually performed via a retrograde approach from the femoral artery. After measurement of the transvalvar gradient, aortic root injection and ventriculography are performed for valve anatomy, degree of insufficiency, and annulus measurement. The valve is dilated by advancing the angioplasty balloon over a guide wire positioned in the left ventricle (Fig. 74.5). Balloon size is chosen to be slightly less than the diameter of the aortic valve annulus. The risk of significant aortic insufficiency increases when the balloon-to-annulus ratio exceeds 110%.
Special considerations apply to critical aortic stenosis in the newborn. This lesion has traditionally been very difficult to treat with high mortality. However, improvements in stratifying patients to appropriate treatment as well as in the treatments themselves have resulted in markedly decreased mortality. The approach to such patients is primarily dependent on left ventricular function and anatomy. When the left ventricle is inadequate to support systemic circulation, patients require treatment as HLHS. Several reports have established criteria for stratification of patients to biventricular repair versus single ventricle palliation. Balloon aortic valvuloplasty is the treatment of choice where left ventricular size (including MV, ventricular volume, and aortic valve sizes) and function are deemed adequate to support systemic circulation. When the left ventricular volume and MV are adequate but the aortic valve hypoplastic, primary Ross procedure may be the optimal strategy. After successful balloon valvuloplasty, ventricular function generally recovers enough to allow discontinuation of ventilatory and intravenous inotropic support within a few days. When this cannot be accomplished, either left ventricular outflow obstruction persists or left heart size and/or function are(is) inadequate. When left ventricular outflow obstruction persists despite a technically
adequate balloon procedure, it is usually the result of annular hypoplasia and may be successfully treated by autograft root replacement. In the latter circumstance, an alternative surgical palliative strategy is the only approach likely to lead to long-term survival. This strategy may be a Norwood operation committing the patient to subsequent single ventricle palliation. Alternatively, a so-called hybrid procedure (see below) may be undertaken. After the hybrid approach, subsequent anatomic
and physiologic evaluation is performed to determine whether the patient is best served with a single or biventricular approach.
adequate balloon procedure, it is usually the result of annular hypoplasia and may be successfully treated by autograft root replacement. In the latter circumstance, an alternative surgical palliative strategy is the only approach likely to lead to long-term survival. This strategy may be a Norwood operation committing the patient to subsequent single ventricle palliation. Alternatively, a so-called hybrid procedure (see below) may be undertaken. After the hybrid approach, subsequent anatomic
and physiologic evaluation is performed to determine whether the patient is best served with a single or biventricular approach.
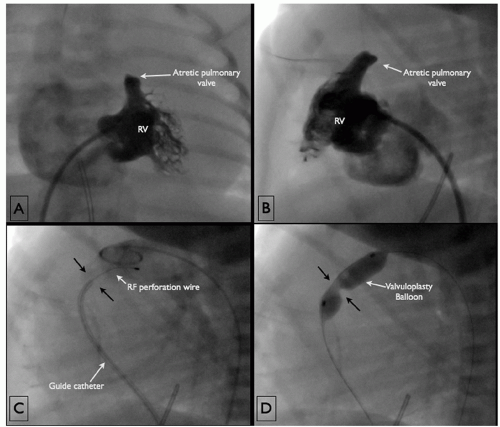 Fig. 74.3. (A) Anteroposterior projection of a right ventricular injection in a newborn demonstrates a tripartite right ventricle with a well-developed infundibulum and valvar pulmonary atresia. (B) A lateral view of the same injection. (C
Get Clinical Tree app for offline access
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree


|