Heart Transplantation
Christopher T. Salerno
Edward D. Verrier
INTRODUCTION
The techniques developed by Norman Shumway and Richard Lower at Stanford University set the stage for heart transplantation to become the therapy of choice for patients with end-stage heart failure. The introduction of transvenous endomyocardial biopsy and the advent cyclosporine immunosuppression dramatically increased patient survival and marked the beginning of the modern era of successful cardiac transplantation. Heart transplantation is now a widely accepted therapeutic option for end-stage cardiac failure, with more than 3,300 procedures performed annually.
RECIPIENT SELECTION
Patients with end-stage heart disease who are being considered as potential candidates for cardiac transplantation should be evaluated by a multidisciplinary committee to ensure an equitable, objective, and medically justified allocation of the limited donor organs. This process should select patients with the greatest chance of postoperative survival and rehabilitation. The primary objective of the recipient selection process is to identify patients with irreversible cardiac disease not amenable to other therapy (optimization of medical therapy, revascularization, ventricular remodeling, valve repair/replacement, biventricular pacing). In our center, the evaluation for a ventricular device is part of the transplant selection process. It is desirable to identify patients who will most likely resume a normal active life and be compliant with the rigorous postoperative medical regimen. Recent successes and the introduction of improved immunosuppression have significantly expanded the eligibility criteria. Patients with New York Heart Association class III or IV symptoms despite optimal medical therapy should be considered for cardiac transplantation. Most patients present with end-stage heart failure due to ischemic heart disease or idiopathic dilated cardiomyopathy. However, the spectrum of known causes of end-stage cardiomyopathy includes infectious (viral), inflammatory, toxic, metabolic, and familial etiologies. Patients selected for cardiac transplantation should have a predicted 2-year survival of <50%. Other less common indications for cardiac transplant include refractory angina, life-threatening arrhythmias, and chronic cardiac allograft rejection. Contraindications to cardiac transplant are primarily based on comorbid illnesses. Examples of widely accepted contraindications include active infection, irreversible hepatic dysfunction, and fixed pulmonary hypertension (pulmonary artery systolic pressure >60 mmHg, transpulmonary gradient >15 mmHg, pulmonary vascular resistance >6 Wood units). Many centers have demonstrated that a left ventricular assist device (LVAD) can be used to reverse fixed pulmonary hypertension and allow patients to become transplant patients. Age in no longer considered to be a contraindication to cardiac transplant.
Preoperative Recipient Evaluation
Evaluation for cardiac transplantation begins with a comprehensive history and physical examination, chest roentgenogram, and lab work including complete blood count, coagulation screen, erythrocyte sedimentation rate, uric acid level, liver function tests, fasting lipid panel, and infectious disease serologies (hepatitis A, B, and C, herpes simplex virus, human immunodeficiency virus, rapid plasma regain, rubella, measles, Toxoplasma). All patients should undergo an exercise test with peak exercise oxygen consumption (peak VO2) measurement. Available studies suggest that patients with peak VO2 <14 ml/min/kg have improved survival and significant functional benefit with transplantation. A right heart cardiac catheterization study should be performed to rule out irreversible pulmonary hypertension. For patients with ischemic cardiomyopathy, coronary angiography should be reviewed or repeated to confirm the inoperability of coronary artery disease. For patients with nonischemic cardiomyopathies and prolonged or atypical symptoms, endomyocardial biopsy should be performed to rule out the possibility of a medically treatable illness.
Most centers also include nutritional labs, thyroid function studies, fasting and postprandial blood sugar, creatinine clearance, 12-lead electrocardiogram, echocardiogram, pulmonary function tests, panel reactive antibody screen, HLA typing, vascular screening exams (abdominal ultrasound, carotid and lower extremity Doppler flow studies), esophagogastroduodenoscopy, psychosocial evaluation, dental evaluation, financial analysis, and screening studies for malignancy (stool guaiac, prostate-specific antigen, mammogram, Papanicolaou smear).
Patients listed for transplantation should be examined routinely for re-evaluation of recipient status. Repeat right heart catheterization is indicated when follow-up echo studies suggest worsening or persistent pulmonary hypertension. Actively listed patients should undergo a repeat right heart catheterization at least twice annually.
DONOR AVAILABILITY AND ALLOCATION
Donor organ availability is the primary factor limiting the application of heart
transplantation. As a result, 20% to 40% of patients on the waiting list may die before transplantation. Organ allocation is based on recipient priority status, time on the waiting list, and proximity. Highest priority is given to local status 1A patients with the longest accrued waiting time. The allocation system is designed to provide the most critically ill patient with a heart while minimizing allograft ischemic time. Although only 25% of patients are classified as status 1 at the time of listing, 48% progress to status 1 by the time of transplantation. The mean waiting period for a status 2 candidate is currently >1 year, whereas status 1 patients wait a mean of 60 days. There is significant variation in waiting times from region to region. Due to this variability, many more patients require an LVAD as a bridge to transplant, in some regions as many as 85% of blood type O patients require an LVAD bridge.
transplantation. As a result, 20% to 40% of patients on the waiting list may die before transplantation. Organ allocation is based on recipient priority status, time on the waiting list, and proximity. Highest priority is given to local status 1A patients with the longest accrued waiting time. The allocation system is designed to provide the most critically ill patient with a heart while minimizing allograft ischemic time. Although only 25% of patients are classified as status 1 at the time of listing, 48% progress to status 1 by the time of transplantation. The mean waiting period for a status 2 candidate is currently >1 year, whereas status 1 patients wait a mean of 60 days. There is significant variation in waiting times from region to region. Due to this variability, many more patients require an LVAD as a bridge to transplant, in some regions as many as 85% of blood type O patients require an LVAD bridge.
Donor Selection
Potential cardiac donors undergo a rigorous screening evaluation. The local organ procurement agency should provide the implanting program with patient’s age, height, weight, gender, blood type, hospital course, cause of death, routine laboratory data, and viral serologies. Additional required data include electrocardiogram, chest roentgenogram, arterial blood gas, and echocardiogram. Coronary angiogram is indicated in a selective manner. The presence of advance donor age (male donors >45 years of age, female donors >50 years of age), risk factors for atherosclerotic coronary artery disease (tobacco abuse, diabetes, significant family history), and occasionally the mechanism of death should be evaluated to determine the need for coronary angiogram. When a member of the transplant team arrives for organ procurement, a secondary screening is performed. This secondary screening allows the recovering surgeon to confirm and review the data presented by the procurement agency. The most important donor screening occurs in the operating room at the time of organ procurement. The heart is examined to identify ventricular or valvular dysfunction previous infarction, coronary atherosclerosis, or myocardial contusion. If direct examination of the heart is unremarkable, the procurement surgeons proceed with donor cardiectomy.
Matching potential recipients with the appropriate donor is based primarily on blood group compatibility and patient size. As a rule, ABO barriers should not be crossed in heart transplantation because incompatibility frequently results in fatal hyperacute rejection. Donor weight should ideally be within 30% of recipient weight except in pediatric patients, where closer size matching is required. If the recipient has elevated pulmonary vascular resistance (>4 Wood units), a larger donor is preferred to reduce the risk of right ventricular failure in the early postoperative period. When the recipient has an LVAD, special consideration should be given to the location of the donor; in these cases, the variable time required to explant the device mandates that the implanting surgeon be able to control the cross-clamp time and organ arrival times. When the percentage (or the panel) reactive antibody (PRA) is ≥10% to 15%, a prospective negative T-cell cross-match between recipient and donor sera is recommended before transplantation. Some centers also insist on a negative B-cell cross-match before transplantation. A positive cross-match is an absolute contraindication to transplantation. A cross-match is always performed retrospectively, even if the PRA is absent or low. Retrospective studies have also demonstrated that better matching at the HLA-DR locus results in fewer episodes of rejection and infection and an overall improved survival. Because of current allocation criteria and limits on ischemic time of the cardiac allograft, prospective HLA matching is not always logistically possible. Many centers have begun to selectively use virtual cross-matching in cases where recipient serum is not available.
There have been recent reports that “extended” donors can be safely used to obtain similar short-term results observed with standard donors. The term “extended” refers to patients with advanced age, high-risk social behavior, limited coronary artery disease, or positive viral serologies. When used, the extended donors are matched to high-risk recipients in which increased risks are acceptable.
DONOR HEART PROCUREMENT
During the secondary donor screening, the location of central and arterial lines should be noted. The location of these lines may affect the conduct of the organ harvest. It is important to continually monitor the donor’s volume status and urine output during the harvest to maintain organ function. The donor is positioned in the supine position and widely prepped from chin to knees. To facilitate both thoracic and abdominal organ recovery, the harvest is begun with a midline incision from sternal notch to pubis, including a median sternotomy. The pericardium is incised and a pericardial well is created. The heart is examined to assure no previously unidentified anomalies exist. After this inspection, the harvesting surgeon should notify the implanting surgeon regarding the suitability of the heart for transplantation.
The heart is then mobilized for cardiectomy. It is important for the harvesting surgeon to know whether any extended lengths of vessels will be necessary to facilitate implantation. For our preferred method of implantation, the bicaval technique, an extended length of superior vena cava (SVC) is required. The SVC is mobilized from the right atrium to the innominate vein. SVC mobilization usually includes dissection of the right pulmonary artery and ligation of the azygous vein. If additional caval length is required, the innominate vein can be harvested en bloc. The inferior vena cava (IVC) is dissected and mobilized circumferentially. Encircling the SVC and IVC with umbilical tapes or heavy suture may assist with retraction during cardiectomy. The aorta is dissected from the pulmonary artery and then isolated with umbilical tape. If hemodynamic instability is encountered during the harvest, clamping of the abdominal aorta at the iliac bifurcation may be helpful. Once the mobilization of the abdominal organs is complete, the donor is given 30,000 units of heparin intravenously. A purse-string suture is placed in the ascending aorta, through which an antegrade cardioplegia catheter is placed. The central venous pressure line is pulled back to beyond the caval-innominate junction. After assurance is given that the abdominal team is ready to proceed, the SVC is clamped or ligated distal to the azygous vein (this avoids sinoatrial nodal injury) (Fig. 63.1).
If the abdominal IVC is vented, the IVC is clamped at the level of the diaphragm. The heart is vented by transecting the left inferior pulmonary vein (or left atrial appendage if the lungs are being concomitantly harvested) and incising the anterior IVC proximal to the clamp. The aortic cross-clamp is applied, and the heart is arrested with cold cardioplegic solution. Adequate perfusion pressure is assessed by palpating the aortic root. The left ventricle should also be monitored to assure that it does not become dilated. Rapid cooling of the heart is achieved with 10 L of topical cold saline (4°C). After a successful arrest, cardiectomy is initiated by completing the transaction of the IVC (Fig. 63.2). The heart is wrapped in an ice-soaked lap pad, and the apex of the heart is elevated. Proceeding from inferior to superior, first on one side and then the other, one divides the remaining intact pulmonary veins and branch pulmonary arteries. When the lungs are being procured for transplantation, the procedure is modified to retain adequate left atrial cuffs and pulmonary arteries for both the heart and
lung implantations. The ascending aorta and SVC are transected last. The underlying disease of the recipient should be taken into account when determining the required length of aorta and SVC.
lung implantations. The ascending aorta and SVC are transected last. The underlying disease of the recipient should be taken into account when determining the required length of aorta and SVC.
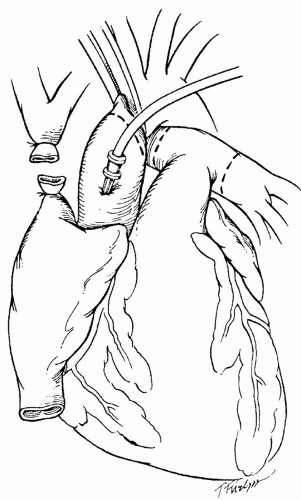 Fig. 63.1. Standard donor cardiectomy. Dotted lines identify aortic and pulmonary artery sites of division. |
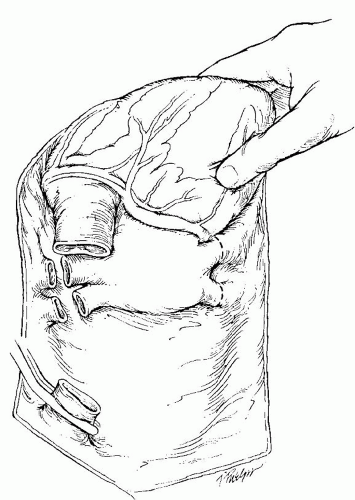 Fig. 63.2. Standard donor cardiectomy. After transaction of the inferior vena cava the heart is retracted cephalad and the pulmonary veins are transected. |
After explanation, the allograft is carefully taken to the back table and placed in a basin of cold saline for inspection and final preparation. The heart is examined for evidence of patent foramen ovale, vascular injuries (or inadequate length), and valvular abnormalities. Any positive findings should be disclosed to the implanting surgeon. The donor heart is then sequentially placed in two sterile bowel bags, each filled with cold saline, a sterile saline-filled air-tight container, and finally a standard cooler of ice for transport.
Several common pitfalls have been identified. Avoiding the following mistakes is vital to successful organ harvest:
Failure to monitor the heart closely during multiorgan dissection.
Failure to heparinize.
Right or left ventricular distension.
Failure to adequately cool the heart during harvest and transport.



