Introduction
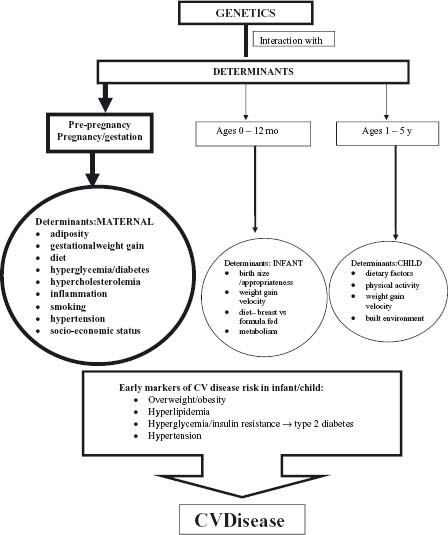
Although cardiovascular (CV) disease presents most commonly in middle-aged adults, it takes many years for the morphologic changes of atherosclerosis to develop and become manifest as clinical CV disease.1,2 Several lines of evidence implicate exposures in fetal life as important determinants of obesity, type 2 diabetes, other CV risk factors and early development of the atherogenic process leading to CV disease. Maternal adiposity, particularly central body fat, and metabolic status at conception, maternal nutritional deprivation and smoking, and exposure of the fetus to an environment of maternal hyperglycemia, hypercholesterolemia, and diabetes, as well as size of the infant at birth, may be key initiating factors for later risk of CV disease (Fig. 20.1). Emerging evidence supports the concept that metabolic programming changes invoked during fetal development may be modified by exposures at other developmental stages in infancy and early childhood.
This chapter will focus on an evaluation of the evidence linking fetal factors to early risk indicators of CV disease to improve insight into the origins of CV disease and to explore how the risk factors at early stages of development can be amenable to modification. This knowledge can lead to effective prevention strategies throughout the life course.
Atherosclerosis development in youth
The earliest morphologic atherosclerotic change, the fatty streak, has been identified in the fetus and is present in many children by three years of age.1 Progression of this collection of lipid-laden macrophages to an intermediate state (raised fatty streak), and eventually to fibrous plaques, occurs in the aorta of many youth by the second decade of life.2–4 This development has been linked to the presence of established risk factors (elevated glucose and blood pressure, excessive weight, dyslipidemia and cigarette smoking) during childhood and adolescence. The prevalence of these risk factors is rising in youth, in part because of the sharp worldwide increases in childhood obesity. In the Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Study and in a similar study in Japan, glycohemoglobin (a marker of dysglycemia), dyslipidemia, cigarette smoking and obesity were related to the extent of atherosclerosis found on postmortem examinations.2,5,6 In the Bogalusa longitudinal study of CV risk factors in youth, the extent of atherosclerosis was also related to blood pressure, and to the clustering of CV risk factors by the second decade of life.7 In addition to the traditional CV risk factors, maternal hypercholesterolemia also influences the development of atherosclerosis in children, independent of the child’s blood cholesterol. This suggests that fetal exposure to hypercholesterolemia can influence atherosclerosis development in the offspring.8 The evidence presented in this chapter highlights the fetal influences on the development of these CV risk factors in youth.
Non-invasive measures of vascular structure (carotid intima-media thickness or cIMT) and function (brachial flow-mediated dilation or FMD), employing ultrasound technology, are used in adults to delineate the extent of atherosclerosis9–11 and predict future coronary artery disease risk.12,13 In young adults followed since early life, childhood low-density lipoprotein (LDL) cholesterol level is an important determinant of cIMT, highlighting the importance of childhood risk factors in the development of adult CV disease. The potential of cIMT as a tool to non-invasively assess atherosclerosis in youth is supported by observations that cIMT is increased in adolescents with markedly elevated LDL cholesterol, and can be reduced by pharmacologic LDL cholesterol lowering in these subjects.14 In addition, cIMT is also greater in adolescents with obesity and type 1 diabetes.15,16 cIMT and FMD have not yet been assessed in younger children in studying the effects of fetal influences, but may be potentially important tools. However, the clinical usefulness of cIMT is dependent on the resolution of the ultrasound equipment in imaging the thinner IMT in children and detection of excess thickness may not be reliable until after puberty.17,18
Fetal health and cardiovascular risk factors and disease: evidence from retrospective studies
The association between fetal health and adult CV disease was shown in early epidemiologic studies linking early childhood data (particularly birth weight) and adult mortality outcomes decades later. These studies provide the epidemiologic evidence that supports the hypothesis that fetal life is important in the development of atherosclerosis and CV disease in adulthood. For the most part, low birth weight was used as a surrogate marker of untoward fetal exposures, but as birth weight is linked to socioeconomic status (which may be reflected in maternal smoking, inadequate healthcare and suboptimal nutrition during pregnancy and which may also influence the child’s health postnatally), the causal linkages between fetal health, birth outcome and later CV disease are difficult to clearly delineate.
Development of CV disease
The link between low birth weight and increased CV disease events in adults, first described in men in the United Kingdom,19 has been reconfirmed in numerous studies, including a study of 15 000 Swedish men and women20 in which 97% of the cohort was tracked at follow-up. Of the 7000 men born in 1915–29 and followed to 1995, risk of CV disease death was highest in those with a birth weight in the lowest quartile compared to the second, third and fourth quartiles (odds ratio (OR), 95% confidence interval (CI): 0.85, 0.71–1.02; 0.67, 0.53–0.84; 0.72, 0.51–1.02, respectively), even when corrected for gestational age and socio-economic status at birth and in adulthood. This relationship was not evident in women in this study. In another study of US women, low birth weight was associated with lower self-reported CV disease events.21 While suggestive, a wide gap in knowledge and information exists between low weight at birth and the subsequent development of CV disease many years later. One problem with these studies is that since CVD presents in mid to late adulthood, assessment of CV disease outcomes requires very long-term follow-up. Adequate follow-up of these cohorts is difficult and uncertainties can arise as to the validity of these findings.
Non-invasive assessment of vascular structure using B-mode ultrasound measuring the cIMT has been useful in identifying markers of atherosclerosis9–11 which serve as surrogates for clinical CV disease in adults.12,13 In a follow-up study of 347 adults aged 49–51 years, multiple health determinants at birth and throughout childhood and traditional CV risk factors in adulthood have been assessed. Although birth weight was inversely related to cIMT, this relationship was no longer significant when adult lifestyle was considered. Early childhood events explained only a small proportion of the variance in cIMT (2.2% in men, 2.0% in women) compared to adult lifestyle (3.4% in men and 7.6% in women) and biologic risk factors in adulthood (9.5% in men and 4.9% in women).22
The causality of the relationship between birth weight and CV disease has also been difficult to establish, as many retrospective historic cohorts were unable to correct for confounding factors including maternal smoking, maternal health and socioeconomic status. Conditions such as pre-eclampsia that result in lower birth weight are themselves associated with CV disease development in the mother, suggesting that conditions that influence the health of both the mother and baby may also confound the relationship of low birth weight23,24 to subsequent CV risk in the fetus. Furthermore, the relative importance of fetal growth restriction relative to other postnatal factors in CV disease development has not been established. Offspring of mothers exposed to extreme undernutrition in the first trimester of pregnancy as a result of food restriction during extreme social upheaval had increased risk of coronary artery disease.25 Suggested potential mechanisms for the influence of reduced fetal growth on adult CV disease include reduced or modified growth and development of the kidneys, vascular smooth muscle, endothelium, heart, pancreas, liver or increased activity of the hypothalamus-pituitary-adrenal axis leading to the development of hypertension, dysglycemia and obesity.
Development of CV risk factors
The observation of a possible relationship between birth weight and risk of subsequent adult CV disease has led to investigations of the relationship between CV risk factors and fetal environmental health. Exposures during the prenatal period of development are also associated with the development of obesity, CV risk factors and dysglycemia in childhood. The accumulation of these risk factors may underlie the increased CV disease risk identified in epidemiologic studies of historic cohorts. Aspects of maternal health including maternal obesity, glycemic environment, nutrition, cigarette smoking and hypercholesterolemia have been related to CV risk factors in the offspring. Furthermore, as with the development of CV disease, fetal growth patterns are also associated with metabolic disturbances. Although not a direct measure of CV disease, long-term follow-up of traditional CV risk factors from childhood to young adulthood affords an opportunity to examine CV risk in established cohorts who are now young adults, and in contemporary pediatric cohorts.
Effect of early growth patterns
In adults, both high and low birth weight have been linked to risk markers for chronic disease from early childhood. Fetal growth restraint, followed by rapid postnatal weight gain and early obesity, is thought to prime the metabolic and endocrine milieu for premature pathology, leading to early onset of chronic diseases such as obesity, hypertension and CV disease. Population and clinical studies demonstrate associations between low birth weight and risk for hypertension,26,27 dysglycemia, insulin resistance28–31 and central obesity in adulthood. Extreme maternal undernutrition during pregnancy has been linked with an increased risk of obesity, hypertension and dysglycemia in the adult offspring of exposed families during the Dutch famine during the Second World War.25,32,33 In contrast, in a recent report, exposure to the Dutch famine was not associated with clustering of CV risk factors or metabolic syndrome,34 and the offspring of mothers exposed to undernutrition during the WWII Leningrad siege showed no such predilection for increased CV risk factor development.35
The relationship between birth weight and adult blood pressure has been explored in several systematic reviews36–38 and despite more than 80 published studies addressing this issue, the conclusions remain controversial. Though an inverse relationship between birth weight and adult blood pressure has been frequently reported, the magnitude of effect is smaller in larger studies (– 1.9 mmHg/ kg birth weight for studies with < 1000 subjects compared to–0.6 mmHg/kg for studies with > 3000 subjects). The discrepancy is in part related to the controversial practice of adjusting adult blood pressure for a factor along its causal pathway, adult body size, resulting in exaggerated findings.39
The relationship between birth weight and adult dysgly-cemia is more complex–described as J-shaped,40 U-shaped41 or inverse.42 Thus, although highest risk is found in those with low and high birth weights in some studies, there is an inverse relation in others.43 Interestingly, in the US Nurses’ Study which initially reported a J-shaped relation, correction for adult BMI resulted in an inverse relationship, which was stronger in those with no family history of type 2 diabetes.40 Type 2 diabetes results from both beta-cell dysfunction and insulin resistance, but the relative importance of these variables in individuals with low birth weight remains unclear. No consistent relationship between birth weight and insulin secretion has been identified43 although it has been vigorously assessed in most studies. Animal studies suggest that nutritional compromise in utero, and low birth weight, may be associated with reduced pancreatic cell mass44 but that reduced insulin sensitivity in muscle or adipocytes45 may only be present concomi-tantly with accelerated postnatal growth.46
The influences of early programming on body composition in adulthood remain controversial. Low birth weight has been associated with central adiposity in some studies47 but not others.48 Most historic cohorts have relied on height, weight and body circumference measurements; few have assessed body fat directly. In several studies that have characterized body composition in adults with dual energy X-ray absorptiometry, a direct relationship between birth weight and lean mass was consistently shown, but inconsistent relationships to body fat were identified.49
Birth weight reflects the responses of the fetus to in utero conditions including maternal health, nutrition and placen-tal function. Conditions that result in low birth weight, e.g. maternal smoking and pre-eclampsia, are associated with adiposity50 and hypertension51 respectively, in the adult offspring. Support for this comes from observations in animal studies of a relationship between low birth weight (due to nutrient restriction in the mother or restricted uteroplacental circulation) and increased adiposity and CV risk factor prevalence in the offspring.52 It remains unclear, however, if the influence of cigarette smoking on adiposity and glucose homeostasis is a result of reduced growth or direct influence of the toxins in cigarette smoke. In an animal model, the offspring of animals exposed to nicotine during pregnancy showed evidence of impaired glucose tolerance53 and loss of pancreatic beta-cell mass.54 Critical windows of effect range from preimplantation to late pregnancy and proposed mechanisms include disturbances in the size and phenotype of organ systems, changes in biochemical thresholds for activity and changes in angiogenesis.55
Interestingly, patterns of accelerated fetal growth as seen in adolescent and adult offspring of mothers with diabetes or overweight are also associated with increased central adiposity, dysglycemia56 and clustering of CV risk factors.57
Effect of glycemic status in utero
Maternal diabetes during pregnancy is consistently associated with greater adiposity and central deposition of body fat in the offspring.28–30 Recently, impaired glucose tolerance (IGT) of pregnancy (often referred to as prediabetes) was also noted to increase risk of greater weight in the offspring.58 Maternal diabetes also enhances the risk of dys-glycemia in the offspring,28–30,56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree