Introduction
Coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) are the most commonly performed major procedures in the world today. Since their introduction, these procedures have been increasingly used for the treatment of coronary artery disease (CAD). In the United States, according to National Center for Health Statistics estimates, nearly 427 000 CABG and 1.2 million PCI procedures were performed in 2004.1 It is estimated that these interventions account for a significant proportion of healthcare expenditures, with the estimated direct and indirect costs of CAD for 2007 being $151.6 billion.1 The immediate risks inherent in these procedures need to be balanced against the expected benefits. Sound clinical judgment and decision making with respect to revascularization options in patients who have CAD require detailed knowledge of both the evidence in support of these procedures and the specific patient subsets that are likely to derive the most benefit.
In general, the broad indications for myocardial revascularization are to:
- improve prognosis (probability of survival)
- improve symptoms
- potentially prevent non-fatal events, such as myocardial infarction (MI), arrhythmias or heart failure.
Over the past four decades, a considerable volume of literature from prospective randomized clinical trials and large national databases has been published on the application and comparisons of various revascularization strategies. These data have helped in formulating well-defined indications for and the selection of appropriate revascularization methods. However, because of rapid growth and refinements in revascularization techniques and technologies, the optimal method of revascularization for numerous patients continues to be a subject of intense debate. In this chapter, we summarize the data from clinical trials and databases, with a view to developing a conceptual framework that allows the application of this information to clinical practice.
The early randomized trials of the 1970s first established CABG as an effective treatment for angina pectoris. These small, prospective, randomized controlled trials included the European Coronary Surgery Study (ECSS),2,3 the Veterans Administration Coronary Artery Bypass Surgery Cooperative Study (VA)4 and the Coronary Artery Surgery Study (CASS).5,6 The trials compared CABG with medical therapy in patients who had stable angina. In a collaborative meta-analysis,3 the primary patient information on 2649 patients enrolled in the evaluated trials was combined to assess outcomes at five and 10 years. Overall, CABG improved survival across all patients by 4.3 months at 10-year follow-up (P = 0.003). Because of the perioperative mortality rate associated with CABG, the mortality rate at one year was not significantly different between the groups, and a net benefit in favor of CABG was not observed for 2–3 years. At 5–7 years, the advantage with CABG increased before it narrowed again at 10–12 years (Fig. 28.1). A significant heterogeneity of treatment effect was observed among various angiographic and clinical subsets. The patients who seemed to derive the most benefit were those with left main CAD (n = 174) (relative risk (RR) 0.32; P = 0.004), multivessel disease (RR 0.58; P < 0.001), and one-or two-vessel disease with proximal left anterior descending coronary artery (LAD) involvement (RR 0.58; P = 0.05). Although the relative benefits were similar regardless of left ventricular (LV) function, the absolute benefit was greater in patients with abnormal LV ejection fraction (EF) because of the higher risk of death in this group. Absolute mortality benefits were also greater among patients with evidence of myocardial ischemia as manifested by severe anginal symptoms or an abnormal exercise test (Table 28.1).
Figure 28.1 Comparison of mortality rates of all studies in meta-analysis of trials of coronary artery bypass grafting (CABG) versus medical treatment. (Reproduced with permission from Yusuf et al3)
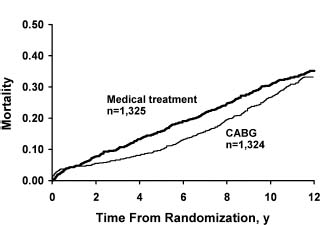
Table 28.1 Results of subgroups with medical therapy and with coronary artery bypass grafting (CABG) at five years
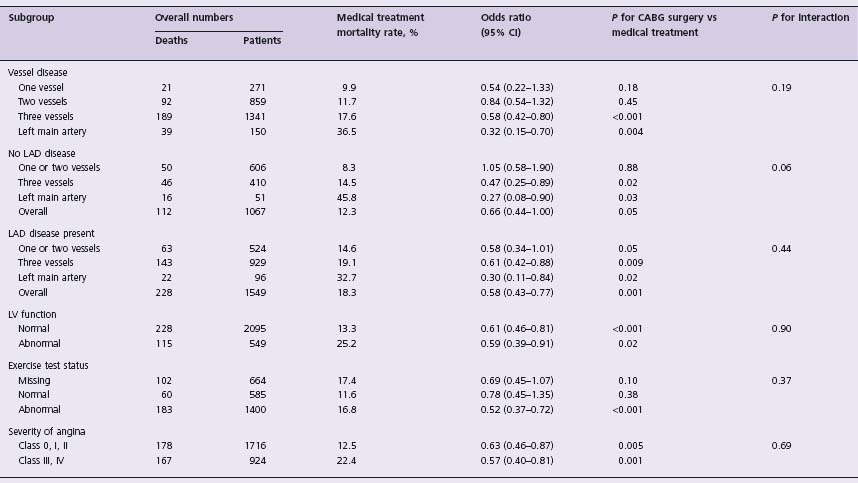
Cl, confidence interval; LAD, left anterior descending artery; LV, left ventricular. Modified from Yusuf et al,3 with permission.
It has been established that, in regard to symptomatic improvement, surgically treated patients have a higher likelihood of staying symptom free than medically treated patients, and use less antianginal medication than medically treated patients at five years.7 However, at 10 years, these differences are not statistically significant. It should be noted, however, that a high degree of cross-over from medical treatment to surgical treatment by 10 years may account for these findings.
In the application of these results to patient care in the current era, several limitations of the trials must be recognized. These early trials tended to include low-risk patients of whom only 20% had an EF of less than 50%. Furthermore, almost all patients were male and between 40 and 60 years of age. Only one trial (CASS) included women and, in this trial, medical therapy was limited to nitrates and beta-blockers. At the time of these trials, aspirin and lipidlowering agents were not routinely prescribed for patients with CAD. In CASS, only 14% of patients received an internal mammary artery (IMA) graft.8 The profound impact of these grafts on survival is now well documented.9,10 The limited use of IMA grafts is a critical limitation of earlier trials of CABG. Also, patients with severe LV dysfunction were excluded from most of the randomized controlled trials. Data on this patient group are largely based on observational studies. In their meta-analysis of randomized trials, Yusuf et al3 found that 40% of the patients assigned to medical therapy in the trials had had surgery by 10 years. These cross-overs in treatment tended to occur in the subgroups at highest risk. Therefore, the true benefits of surgery, particularly in the high-risk groups, are likely underestimated. In the recent BARI 2D trial performed in patients with type 2 diabetes and stable coronary artery disease, by 5 years of follow-up there were fewer major cardiovascular events among CABG patients than among those receiving only optimal medical therapy.40a
Patients older than 75 years are increasingly undergoing CABG, and population-based rates estimates are higher than for patients younger than 75 years.2,11 Despite this profile of increasing risk, operative mortality rates have continued to decline.12 Since the early trials, advances have been substantial in the medical, percutaneous, and surgical management of CAD. Beta-blocker use is now more widespread, and the introduction of newer antiplatelet agents, statins, and angiotensin-converting enzyme inhibitors has revolutionized the medical management of these patients. Percutaneous revascularization techniques are common and continue to evolve with improved technology, experience, and the use of drug-eluting stents (DES) and adjuvant antiplatelet therapies. The operative and perioperative management of patients who undergo bypass surgery has also improved with increased use of arterial grafting, less invasive procedures, and improved attention to secondary prevention. Although the main messages obtained from the early trials are still relevant today, estimates of the degree of risks and benefits are likely to be different today from those suggested by these studies.
The effectiveness of percutaneous coronary intervention
Since the introduction of percutaneous transluminal coronary angioplasty (PTCA) in the 1970s as a treatment for single-vessel CAD, it has become one of the most commonly performed procedures. During the past three decades, PTCA procedures have been applied to multivessel CAD and even to left main CAD. Initial trials of PTCA compared balloon angioplasty with medical therapy. However, with the advent of coronary artery stents, subsequent studies saw an increasing use of these devices.
Balloon angioplasty versus medical therapy (see also Chapter 26)
A meta-analysis of six prospective, randomized clinical trials comparing balloon angioplasty with medical therapy included 1904 patients with nonacute CAD.13,14 Among the low-risk patients with symptomatic CAD (Canadian Cardiovascular Society (CCS) angina class II or greater; average mortality rate < 1% per year), balloon angioplasty clearly provided superior control of angina pectoris, although it was associated with an increased need for subsequent CABG. There was no significant impact on subsequent death, MI or subsequent balloon angioplasty. These data suggested that balloon angioplasty was indicated when the desired level of anginal relief and physical activity could not be achieved with medical therapy alone and that prophylactic balloon angioplasty could not be recommended for the treatment of CAD in the absence of angina or ischemia.14 However, most of these studies were undertaken before 1997. Balloon angioplasty was plagued by restenosis, with a need for repeat procedures in up to 34% of patients at one year.15
Bare metal stents
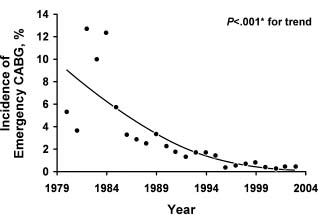
The high incidence of restenosis after balloon angioplasty was initially addressed with the introduction of bare metal stents (BMS). Introduced for the treatment of coronary dissections after balloon angioplasty, BMS were rapidly adopted by clinicians for routine use during balloon angioplasty, thus leading to immediate improvements in procedural safety and success. In particular, the rate of emergency CABG decreased from about 5% to less than 1%, and angiographic success became routine and independent of lesion morphologic factors16 (Fig. 28.2). The short-term therapeutic effects of these stents have been evaluated in several trials,17,18 and although restenosis was significantly decreased, it continued to be a substantial clinical problem.17 In a meta-analysis based on 29 trials involving 9918 patients, there was no evidence of a difference between routine coronary stenting and standard PTCA in terms of deaths or MI (odds ratio (OR) 0.90, 95% confidence interval (CI) 0.72–1.11) or the need for CABG (OR 1.01, CI 0.79–1.31). Coronary stenting decreased both the rate of restenosis (OR 0.52, CI 0.37–0.69) and the need for repeated PTCA (OR 0.59, CI 0.50–0.68).19 It is important to note that these trials did not include higher-risk lesions treatable with stenting but not with PTCA alone.
Figure 28.2 Incidence of emergency coronary artery bypass grafting (CABG) over time after percutaneous coronary intervention procedures, 1979–2004. *Armitage test for trend. (Reproduced with permission from Yang et al16)
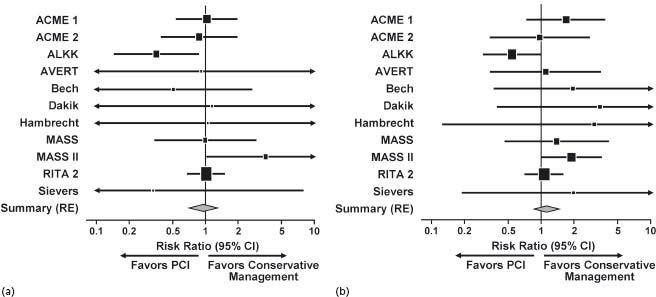
Several studies have compared PCI performed with or without stents with medical therapy in patients who have stable CAD. A meta-analysis of 11 randomized controlled trials of PCI versus medical therapy included a total of 2950 patients with non-acute CAD.20 The use of stents in the trials of this meta-analysis was variable (range, 30–100%). There was no substantial difference between initial PCI and initial medical strategies with regard to mortality rate, cardiac death, fatal MI or non-fatal MI20 (Fig. 28.3).
Figure 28.3 Results of meta-analysis comparing percutaneous coronary intervention (PCI) with conservative management. (a) Death. (b) Cardiac death or myocardial infarction. ACME, Angioplasty Compared to Medicine Evaluation; ALKK, Working Group of Leading Hospital Cardiologists; AVERT, Atorvastatin Versus Revascularization Treatment; CI, confidence interval; MASS, Medicine, Angioplasty or Surgery Study; RE, random effects; RITA, Randomized Intervention Treatment of Angina. (Reproduced with permission from Katritsis and Ioannidis.32)
The most recent comparison of PCI and medical therapy was the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial.21 The investigators randomly assigned 2287 patients with stable CAD to receive either optimal medical therapy alone or PCI with optimal medical therapy on the basis of carefully selected clinical and angiographic criteria. At a median follow-up of 4.6 years, the cumulative frequency of the primary endpoint of death or non-fatal MI was 19.0% in the PCI group and 18.5% in the medical therapy group (P = 0.62).
In conclusion, PCI and medical therapy appear to lead to similar outcomes in rates of death and MI in patients with stable CAD. PCI with BMS is consistently associated with lower rates of angina and subsequent revascularization compared with medical therapy. In patients who have characteristics similar to those of patients enrolled in the randomized controlled trials, either strategy, medical therapy or PCI, is appropriate.
Drug-eluting stents
Despite the introduction of BMS, the chief limiting factor of percutaneous revascularization continued to be restenosis. Consequently, numerous agents have been used as stent coatings in the hope of preventing thrombosis, neointimal proliferation, and subsequent restenosis. Currently, all FDA-approved DES are a device-polymer-drug combination. Several randomized clinical trials have found that these stents are efficacious in decreasing the rates of restenosis and revascularization.22–37
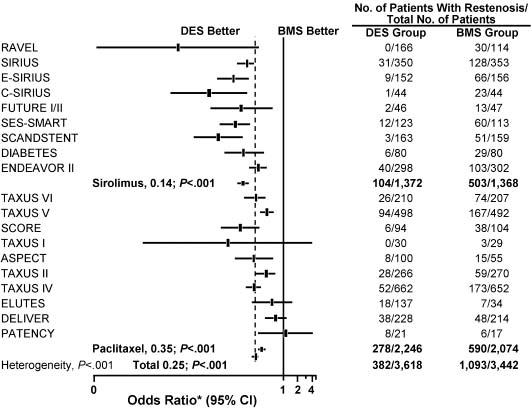
In a meta-analysis of 19 randomized controlled trials that included 8987 patients and compared DES with BMS, the overall mortality rate was not significantly different between the treatment groups (0.9% with DES vs 1.2% with BMS; P = 0.92).38 The overall adjusted rate for angiographic restenosis was 10.6% in the DES group versus 31.8% in the BMS (control) group (P < 0.001) (Fig. 28.4). The overall adjusted rate for angiographic target-lesion revascularization was 6.2% in the DES group versus 16.6% in the control group (P < 0.001). The Q-wave MI-adjusted percentage was 1.1% with DES versus 0.8% with BMS (P = 0.33).
Figure 28.4 Angiographic restenosis found in meta-analysis of trials comparing the drug-eluting stent (DES) with the bare metal stent (BMS). ASPECT, Asian Paclitaxel-eluting Stent Clinical Trial; C-SIRIUS, Canadian Sirolimus-coated Balloon-expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions; DELIVER, Non-polymer-based Paclitaxel-coated Coronary Stents for the Treatment of Patients With De Novo Coronary Lesions; DIABETES, Diabetes and Sirolimus-eluting Stent Trial; ELUTES, European Evaluation of Paclitaxel-eluting Stent; ENDEAVOR, Randomized, Controlled Trial of the Medtronic Endeavor Drug (ABT-578)-eluting Coronary Stent System Versus the Taxus Paclitaxel-eluting Coronary Stent System in De Novo Native Coronary Artery Lesions; E-SIRIUS, European Sirolimus-coated Balloon-expandable Stent in the Treatment of Patients with De Novo Native Coronary Artery Lesions; FUTURE, First Use to Underscore Reduction in Restenosis With Everolimus; PATENCY, Paclitaxel-coated Logic Stent for the Cytostatic Prevention of Restenosis; RAVEL, Randomized Study With Sirolimus-coated BX Velocity Balloon-expandable Stent in the Treatment of Patients with De Novo Native Coronary Lesions; SCANDSTENT, Randomized Multicentre Comparison of Sirolimus Versus Bare Metal Stent Implantation in Complex Coronary Lesions; SCORE, Study to Compare Restenosis Rate Quest and Quads QP2; SES-SMART, Randomized Comparison of a Sirolimus-eluting Stent and a Standard Stent in the Prevention of Restenosis in Small Coronary Arteries; SIRIUS, Sirolimus-eluting Balloon-expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions; TAXUS, Treatment of De Novo Coronary Disease Using a Single Paclitaxel-eluting Stent. * OR 0.25 (95% CI 0.22–0.29); P < 0.001. (Modified with permission from Roiron et al38)
There has been a tendency to regard restenosis as a benign process. However, emerging data seem to challenge this notion, largely because a substantial fraction of patients who have restenosis present with acute coronary syndromes. In a study of 4503 consecutive patients treated with at least one BMS, 667 patients had 836 occurrences of clinical restenosis.39 The cumulative incidence of at least one restenosis was 9.6% at one year, 13.9% at five years, and 18.1% at 10 years. Among all patients, the 10-year incidence of restenosis according to mode of presentation was stable angina in 9.0%, unstable angina in 7.4%, MI in 2.1%, and other unstable presentations, such as decompensated heart failure or ventricular arrhythmia, in 0.4%. Among 87 patients with an MI presentation of restenosis, non-ST segment elevation MI (NSTEMI) was seen in 87% and ST segment elevation MI (STEMI) was seen in 13%. Presentation of MI with restenosis was associated with a significantly greater mortality risk than either a presentation of MI with no restenosis (hazard ratio (HR) 2.37, 95% CI 1.72–3.27; P < 0.001) or a non-MI presentation of restenosis (HR 2.42, 95% CI 1.68–3.47; P < 0.001). Interestingly, there was no significant difference in long-term survival among patients with no restenosis compared to patients with a non-MI presentation of restenosis (HR 0.99, 95% CI 0.85–1.14; P = 0.85).
Safety
There have been recent concerns about the long-term safety of DES. However, recent pooled analyses of the trials of sirolimus-eluting stents and paclitaxel-eluting stents show no difference in the long-term rates of death, MI or stent thrombosis.40–42 In one analysis, the risk of stent thrombosis increased after one year with the use of a DES.43 This did not translate into an increased frequency of death or of MI. Data from the Swedish Coronary Angiography and Angioplasty Registry (SCARR)44 seemed to suggest an increased incidence of both death and MI on long-term follow-up of patients with DES. More recent data from SCARR showed no differences in death or MI.45 In the clinical trials, the enrolled subjects were patients with PCI who were at relatively low risk.
A 2006 review of patient data in the American College of Cardiology (ACC) National Cardiovascular Data Registry found that 24% of procedures involving DES were for off-label indications (i.e. STEMI, in-stent restenosis, saphenous vein graft stenosis, and chronic total occlusion).46 Of the 3323 patients in the Evaluation of Drugeluting Stents and Ischemic Events (EVENT) registry, 1817 (54.7%) patients had at least one off-label characteristic on the basis of FDA-approved indications for sirolimus and paclitaxel-eluting stents. During the index hospitalization, the composite clinical outcome occurred in 198 (10.9%) patients in the off-label group and 76 (5.0%) patients in the on-label group (adjusted OR 2.32, 95% CI 1.75–3.07; P < 0.001). At one year, the composite clinical outcome occurred more often in the off-label group (309 (17.5%) patients) than in the on-label group (131 (8.9%) patients) (adjusted HR 2.16, 95% CI 1.74–2.67; P < 0.001). In-stent thrombosis also occurred more frequently among patients in the off-label group than among patients in the on-label group during the initial hospitalization (8 (0.4%) patients vs 0 patients) and at one year (29 (1.6%) patients vs 13 (0.9%) patients) (adjusted HR 2.29, 95% CI 1.02–5.16; P = 0.05).47
A higher prevalence of complex interventions that use DES in subsets of patients at relatively higher risk will in all likelihood translate into increased event rates in DES subgroups in registry studies. For all these high-risk groups, compared to low-risk groups, event rates are increased after any treatment. The available data on the use of DES in specific complex subsets of patients and lesions seem to suggest that these stents may be superior to BMS in these circumstances.48,49 Randomized clinical trials are needed to fully define the role of DES in such patient groups.50
Comparisons of percutaneous coronary intervention versus coronary artery bypass graft
Considerable controversy has existed, and continues to exist, about the relative benefits of PTCA compared with CABG as initial treatment strategies. Up to 15 randomized trials comparing PCI, with or without stents, with CABG have been published and were reviewed extensively by both Eagle et al51 and Hoffman et al.52 Recently, the 10-year results of the Bypass Angioplasty Revascularization Investigation (BARI), the largest of the randomized trials of PTCA versus CABG, were published.52 In this trial, 1829 symptomatic patients with multivessel CAD were randomly assigned to initial treatment with PTCA or CABG and were observed for an average of 10.4 years. The 10-year survival was 71.0% for the PTCA group and 73.5% for the CABG group (P = 0.18). At 10 years, the PTCA group had substantially higher subsequent revascularization rates than the CABG group (76.8% vs 20.3%; P < 0.001), but angina rates for the two groups were similar. In the subgroup of patients with no treated diabetes mellitus, survival rates were nearly identical for the PTCA group compared with the CABG group (77.0% vs 77.3%; P = 0.59). In the subgroup with treated diabetes, the group assigned CABG had higher survival rates than the group assigned PTCA (57.8% vs 45.5%; P < 0.03).
Another recent publication comparing CABG, PCI, and medical therapy was based on the five-year outcomes of the Medicine, Angioplasty or Surgery Study-II (MASS-II)53,54
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


