Magnitude of the problem
Throughout the last few decades, non-cardiac surgery has made substantial advances in treating diseases and improving patient quality of life, and as a result, the number of patients undergoing non-cardiac surgery is growing. A recent study used surgical data from 56 countries around the world to determine that globally there are over 230 million major surgical procedures undertaken annually.1 The fact that cardiac and pediatric surgery only account for a minority of major surgical cases suggests that over 200 million adults undergo major non -cardiac surgery annually.
Non-cardiac surgery is associated with an increased risk of major vascular complications (i.e. vascular death, non-fatal myocardial infarction, non-fatal cardiac arrest, and non-fatal stroke). A recent review found only one study, by Lee and colleagues,2 that evaluated the incidence of major perioperative vascular complications in a prospective cohort that fulfilled the following criteria: more than 300 patients, the patients were relatively unselected (i.e. not restricted to patients referred to internal medicine or to patients with or at high risk of coronary artery disease), not restricted to a specific type of surgery (e.g. orthopedics), and required patients to have at least one measurement of a cardiac biomarker or enzyme after surgery.3 This study suggests that major perioperative vascular events occur in 1.4% (95% confidence interval (CI) 1.0 – 1.8%) of adults undergoing elective non-cardiac surgery. Conservative estimates suggest that at least half of the 200 million adults undergoing non-cardiac surgery are in an at-risk age group.4 These data together with the data from the Lee study suggests that worldwide, 1 – 1.8 million adults suffer a major perioperative vascular complication annually.
There is concern, however, that this is a substantial underestimation of the actual incidence. The recent Peri-Operative ISchemic Evaluation (POISE) trial included 8351 patients from 190 hospitals in 23 countries in a randomized controlled trial comparing the effects of a beta-blocker relative to placebo among patients undergoing non -cardiac surgery.5 The incidence of major perioperative vascular complications in the POISE trial was more than three times higher than what was predicted by the Revised Cardiac Risk Index, which was developed from the study by Lee and colleagues. This suggests the possibility that the current incidence of major perioperative vascular complications is substantially higher than the estimate from the Lee study. Several limitations associated with the Lee study support this position. Lee et al did not include stroke as an outcome, and excluded patients undergoing emergency surgery.2 Emergent cases represent about 10% of non-cardiac surgeries6 and patients undergoing emergency surgery are at higher risk of major perioperative vascular events than patients undergoing elective surgery (odds ratio (OR) 2.6, 95% CI 1.2 –5.6).7 The data from Lee and colleagues are over 15 years old. Considering that patients with coronary artery disease are living longer and there has been a practice pattern shift towards advanced care for the elderly (including surgery), this suggests that older patients with high burdens of coronary artery disease are now surviving to develop other conditions that require an operation and these elderly patients are undergoing surgery.
These limitations support the finding in the POISE trial, and this suggests that the current worldwide incidence of adults suffering a major perioperative vascular complication in the first 30 days after surgery is probably in the range of 3 – 5.4 million annually. This is in the range of the global incidence of new patients acquiring human immunodeficiency virus (HIV) annually,8 and identifies major perioperative vascular complications as a similarly common and important public health problem.
Need for preoperative risk prediction
Accurate estimation of perioperative vascular risk in patients undergoing non-cardiac surgery is important to guide perioperative management and to allow patients and physicians to make informed decisions about the appropriateness of surgery. The majority of non-cardiac surgeries are elective procedures. An accurate estimate of risk facilitates patient and physician decision making. For example, if an elderly female with multiple risk factors undergoing hip arthroplasty for osteo-arthritis was accurately informed that her risk of a major perioperative vascular event was 10 – 12%, she may decide to defer surgery for a year, living with suboptimal quality of life until after her grand-daughter’s wedding. Further, accurate perioperative cardiac risk prediction can inform management decisions (e.g. whether to delay or cancel surgery or type of anesthetic approach) and monitoring decisions (e.g. whether a patient should go to a telemetry unit after surgery).
Clinical risk prediction
Researchers have developed two types of clinical models – generic and Bayesian – to estimate perioperative cardiac risk in patients undergoing non-cardiac surgery. The generic risk models estimate a patient’s risk of a perioperative cardiac event through determination of how many predictors of risk (e.g. history of coronary artery disease, diabetes, emergency surgery) an individual patient fulfills. The Bayesian risk models modify the average cardiac event rate for a specific surgery or group of comparable surgeries (pretest probability) through use of a patient’s individual index score (likelihood ratio), which is based upon how many predictors of risk (e.g. history of coronary artery disease, diabetes) an individual patient fulfills. The result provides an estimate of the patient’s risk of a perioperative cardiac event (post-test probability).
Four clinical models developed to predict perioperative cardiac events fulfill the following criteria: assessed in >300 patients, validated at least within the original study, and not restricted to a specific type of non-cardiac surgery (e.g. vascular surgery).2,7,9,10 Table 5.1 presents information on the studies that developed these clinical models and Table 5.2 presents their scoring systems. All studies had a small to moderate number of events and were underpowered to assess the independent effect of each variable given the number of variables assessed. These clinical models were designed to predict cardiac outcomes of varying importance, and no model included stroke. Table 5.3 presents the studies that have evaluated the predictors of stroke in patients undergoing non-cardiac surgery.11–15 Although there are few studies and most observed few events, they do suggest overlap between the predictors of stroke and major cardiac events in patients undergoing non-cardiac surgery. The study with the most number of perioperative strokes (i.e. 61 strokes) suggests that perioperative stroke is a serious outcome (i.e. 18% died and 31% were care dependent at discharge).15
The Revised Cardiac Risk Index is the simplest of the models to use and is more predictive than the Original Cardiac Risk Index.2,7 Of the two Bayesian models, the Veterans Affairs Model is simpler to use and the one study that compared these two models suggested the Veterans Affairs Model was as predictive as the Modified Cardiac Risk Index.7 Although several studies have compared the predictive accuracy of the generic and Bayesian risk models,2,7,9,16,17 only two have used pretest probabilities in the Bayesian models based upon contemporary data from the hospitals included in the studies.7,9 The most recent of these two studies demonstrated superior prediction capabilities of the Bayesian risk models.7
Table 5.1 Studies that have developed clinical models to predict cardiac events in patients undergoing non-cardiac surgery
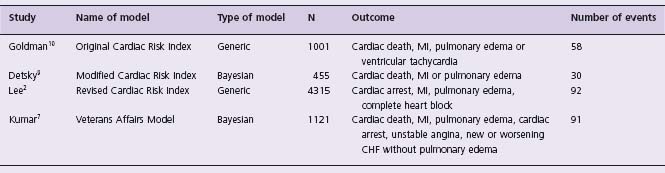
MI, myocardial infarction; CHF, congestive heart failure.
Table 5.2 Clinical models to predict cardiac events in patients undergoing non-cardiac surgery
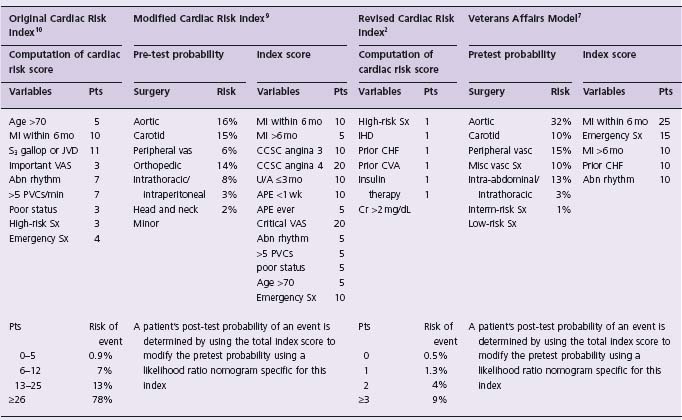
abn rhythm, rhythm other than sinus or premature atrial contractions on last preoperative electrocardiogram; APE, alveolar pulmonary edema; CCSC, Canadian Cardiovascular Society Class; CHF, congestive heart failure; Cr, creatinine; CVA, stroke or transient ischemic attack; IHD, ischemic heart disease; JVD, jugular vein distension; mo, months; poor status, PO 2 < 60, PCO 2 > 50 mmHg, K < 3.0, HCO3 < 20 meq/L, blood urea nitrogen > 50, creatinine > 3.0 mg/dL, abnormal serum glutamic oxalacetic transaminase, signs of chronic liver disease, or patient bedridden from non-cardiac causes; Pts, points; PVCs, premature ventricular contractions; U/A, unstable angina; vasc, vascular; VAS, valvular aortic stenosis; low-risk Sx, low-risk surgery (e.g. ophthalmology, maxillofacial, plastic, low-risk orthopedic, urologic, general surgery and non-thoracotomy thoracic procedures); Interm-risk Sx, intermediate risk surgery (e.g. neurosurgery, ENT surgery, major orthopedic surgery); high-risk Sx, high-risk surgery (e.g. intraperitoneal, intrathoracic, or aortic surgery); misc vasc Sx, miscellaneous vascular surgery (e.g. all lower extremity amputations, arteriovenous access procedures).
Despite this finding, the current predictive accuracy of the Modified Cardiac Risk Index and the Veterans Affairs Model is uncertain because there is no high-quality study that has established contemporary complication rates for individual surgeries or groups of comparable surgeries, and it is unknown if contemporary complication rates at one institution are applicable to another institution. Further, it is unclear whether the Bayesian models would demonstrate superior prediction capabilities if the generic risk models included more specific surgeries or groups of comparable surgeries as risk factors, because it is likely that the type of surgery a patient undergoes influences the probability of a vascular complication.
The Vascular events In non-cardiac Surgery patIents cOhort evaluatioN (VISION) Study is a prospective cohort study of 40 000 patients undergoing non-cardiac surgery in several countries around the world (ClinicalTrials.gov, number NCT00512109). A primary objective of this study is to develop a current clinical risk estimation model for predicting major perioperative vascular complications. Until further studies like the VISION Study are complete, physicians need a practical clinical risk index. The Revised Cardiac Risk Index from the Lee study is the simplest to use in clinical practice, and several studies have validated the factors included in the index as independent predictors of major perioperative vascular complications.18 The Revised Cardiac Risk Index consists of six equally weighted risk factors: high-risk surgery (intraperitoneal, intrathoracic or suprainguinal vascular surgery), history of ischemic heart disease, history of congestive heart failure, history of cerebrovascular disease (i.e. stroke or transient ischemic attack), use of insulin therapy for diabetes, and a preoperative serum creatinine >175μmol/L (>2.0mg/dL). Table 5.4 presents data from the original study and demonstrates the percentage risk of patients suffering a major perioperative cardiac event (i.e. cardiac death, non-fatal myocardial infarction, and non-fatal cardiac arrest) and the corresponding 95% confidence intervals, based on the number of risk factors met. As stated above, the observed event rate in the recent large international POISE trial demonstrated an observed event rate that was three times higher than that predicted by the Revised Cardiac Risk Index. Therefore, physicians may want to double or triple the estimates from Table 5.4 until further data are available.
Table 5.3 Predictors of stroke in patients undergoing non-cardiac surgery
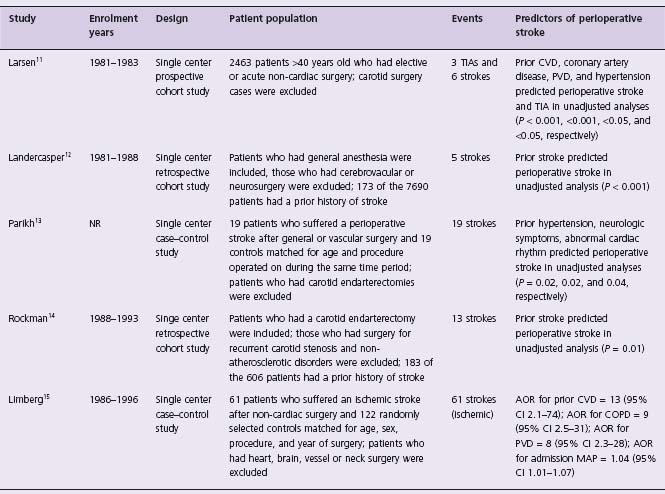
AOR, adjusted odds ratio; CVD, cerebrovascular disease; COPD, chronic obstructive pulmonary disease; PVD, peripheral vascular disease; MAP, mean arterial pressure; TIA, transient ischemic attack; NR, not reported; neurologic symptoms, optic migraine, transient ischemic attack, and stroke.
Non-invasive cardiac stress testing
Although clinical risk indices help to identify patients at risk of a major vascular complication, they underestimate risk in a substantial proportion of patients.3,17 This likely occurs because for a prolonged period of time prior to undergoing surgery, many patients have limited mobility (e.g. patients with arthritis, peripheral vascular disease, cancer). Some of these patients have underlying cardiac disease but are not active enough to experience symptoms. Although it is likely that researchers will develop more accurate clinical risk indices, it is also likely that a substantial proportion of patients undergoing non-cardiac surgery (e.g. patients with limited mobility, diabetes) will require further evaluation beyond clinical symptoms to optimize their risk assessments.
To address this problem researchers have assessed the prognostic capabilities of non-invasive cardiac stress tests (e.g. stress echocardiography, nuclear scintigraphy imaging), prior to non-cardiac surgery.3 A recent meta-analysis evaluating these two tests demonstrated that they have only moderate negative likelihood ratios (stress echocardiography 0.23 and stress perfusion imaging 0.44), and more than a third of the patients who suffered a major perioperative cardiovascular event had a negative test result.19 The studies that directly compared these tests suggested there was no difference between the two tests regarding their positive likelihood ratios. When either of these tests had a moderate-to-large defect it was associated with a positive likelihood ratio of 8; however, this test result was only present in a small proportion of patients (i.e. < 15% of the patients undergoing one of these tests).
Table 5.4 Perioperative cardiac risk estimation based upon predictors in the R evised C ardiac risk Index
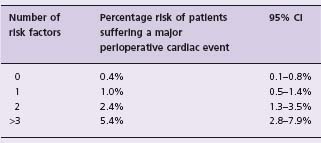
This table is reproduced with permission from Devereaux et al.3 Risk factors = high-risk surgery (intraperitoneal, intrathoracic or suprainguinal vascular surgery), history of ischemic heart disease (defined as a history of myocardial infarction, positive exercise test, current complaint of ischemic chest pain or nitrate use, or ECG with pathologic Q waves; patients with prior coronary bypass surgery or angioplasty were included only if they had such findings after their procedure), history of congestive heart failure (defined as a history of heart failure, pulmonary edema or paroxysmal nocturnal dyspnea; an S3 gallop or bilateral rales on physical examination, or a chest radiograph showing pulmonary vascular resistance), history of cerebrovascular disease (i.e. stroke or transient ischemic attack), use of insulin therapy for diabetes, and a preoperative serum creatinine > 175 μ mol/L (> 2.0 mg/dL).
Major perioperative cardiac event = cardiac death, non-fatal myocardial infarction or non-fatal cardiac arrest; note that this table does not include postoperative cardiogenic pulmonary edema and complete heart block, which were included as outcomes in the Lee index.
These data likely represent a best-case scenario for these tests because the results report the direct association between the non-invasive cardiac stress test and the risk of a major perioperative cardiovascular outcome. Because of the cost and time associated with these tests, the more relevant data are whether these non-invasive cardiac stress tests provide additional predictive value, beyond clinical variables, for the occurrence of major perioperative cardiovascular events. Most of the studies have not assessed whether these non-invasive cardiac stress tests provide independent prognostic information. The studies that have undertaken multivariable analysis are unreliable because they did not include all the known independent clinical variables or the analysis had too few events for the number of variables assessed.20–24 The results and the limitations of these data leave considerable uncertainty as to the role of non-invasive cardiac stress testing prior to non-cardiac surgery.
Preoperative BNP and NT-pro BNP measurement
Ventricular myocytes secrete brain natriuretic peptide (BNP), a prohormone, and its inactive cleavage product N-terminal fragment of the prohormone (NT-proBNP) into the blood in response to myocardial stretch and ischemia.25,26 Plasma BNP and NT-proBNP are powerful predictors of death and major adverse cardiovascular events in patients with stable coronary artery disease, acute coronary syndromes, and congestive heart failure.27–31 Table 5.5 reports six studies that have evaluated the independent association between a preoperative BNP or NT-proBNP measurement and major perioperative cardiovascular events.32–37 All studies suggest that an elevated preoperative BNP or NT-proBNP measurement is a strong independent predictor of a major perioperative cardiovascular event. Although there are limitations to these studies regarding the number of events, variations in the outcomes and thresholds evaluated, and the width of the confidence intervals, these results are encouraging. A large substudy (10 000 patients) of the VISION Study currently under way is evaluating the prognostic capabilities of preoperative NT-proBNP and will provide further insights into whether NT-proBNP, a relatively cheap and easily accessible test compared to the non-invasive cardiac stress tests, can enhance perioperative clinical risk predictions.
Table 5.5 Adjusted association between pre-operative BNP/NT-p ro BNP level and perioperative cardiovascular event
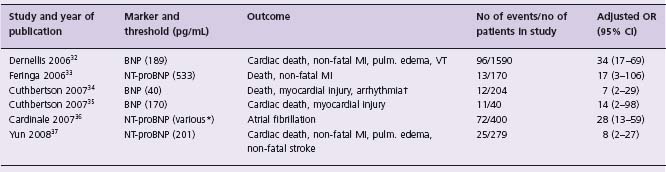
MI, myocardial infarction; pulm., pulmonary; VT, ventricular tachycardia; ACS, acute coronary syndrome.
* Authors employed six age-and sex-dependent thresholds;
† resulting in hemodynamic compromise or requiring intervention.
Perioperative myocardial infarctions
Considering the major perioperative vascular complications (i.e. vascular death, non-fatal myocardial infarction, non-fatal cardiac arrest, and non-fatal stroke) myocardial infarction is the most common event. For example, in the POISE trial 1.6% of patients suffered a vascular death, 0.7% suffered a stroke, 0.5% suffered a non-fatal cardiac arrest, and 5.0% suffered a myocardial infarction in the first 30 days.5
Pathophysiology of perioperative myocardial infarction
Rupture of atherosclerotic plaque with superimposed arterial thrombosis constitutes the underlying pathophysiology in the majority of non-operative myocardial infarctions.38 Between 64% and 100% of patients with non-operative myocardial infarctions have coronary artery plaque fissuring39,40 and 65 – 95% have an acute luminal thrombus.40–44
A commonly proposed mechanism of perioperative myocardial infarction relates to myocardial oxygen supply demand mismatch.45 Fluid shifts, catecholamine surges, hypotension, anemia, pain, hypothermia, and hypoxia can occur during and after major non-cardiac surgery and transiently increase myocardial oxygen demand.3 In coronary vessels with high-grade stenoses or occlusions, the supply response may be limited, resulting in supply/demand mismatch myocardial infarction. Consistent with this hypothesis, two small retrospective autopsy studies (<70 patients in total) that reported the coronary pathology of patients who suffered a fatal perioperative myocardial infarction revealed that two-thirds of patients had significant left main or three-vessel coronary artery disease.46,47 Most of the patients did not exhibit plaque fissuring and only about one-third had an intracoronary thrombus. Although these data require cautious interpretation because the timing of the autopsies relative to the myocardial infarctions may have allowed resolution of intracoronary thrombus, these data suggest that a substantial proportion of fatal periop-erative myocardial infarctions may be secondary to an increase in oxygen demand in the setting of a fixed coronary artery stenosis.
Although POISE does not provide direct evidence regarding the pathophysiology of perioperative myocardial infarction, some of the findings challenge the theory that perioperative myocardial infarction result from an increase in oxygen demand in the setting of fixed coronary artery stenoses.5 In POISE, perioperative beta-blockers prevented myocardial infarctions (beta-blockers 4.2% v placebo 5.7%; hazard ratio (HR) 0.73; 95% CI 0.60 – 0.89; P = 0.0017) but increased clinically significant hypotension (systolic blood pressure less than 90 mmHg that someone intervened upon; beta-blockers 15.0% vs placebo 9.7%; HR 1.55; 95% CI 1.38 – 1.74; P < 0.0001). Clinically significant hypotension had the largest population-attributable risk for perioperative death and perioperative stroke but appeared to have minimal impact on the risk of perioperative myocardial infarction. Because myocardial oxygen supply is critically dependent on maintaining coronary blood flow during diastole, hypotension of sufficient severity to cause stroke and death would be expected to further compromise coronary perfusion in patients with pre-existing fixed coronary artery stenosis, thereby increasing the risk of perioperative myocardial infarction with beta-blockers rather than reducing the risk. Therefore, the POISE results suggest the possibility that other mechanisms beyond increased oxygen demand in the setting of a fixed coronary artery stenosis are involved in the pathogenesis of perioperative myocardial infarction.
An additional or alternative mechanism of perioperative myocardial infarction is that the acute stress of surgery and mechanical tissue injury induce a hypercoagulable state that increases the risk of coronary thrombus formation at the sites of a fissured plaque or low coronary flow. This mechanism might also explain the reduction in perioperative myocardial infarction seen with beta-blockers in POISE because sympathetic hyperactivity promotes hypercoagulability by upregulating coagulation and platelets and downregulating fibrinolysis, and this effect can be suppressed by beta-blocker therapy.48–51
Consistent with the thrombosis hypothesis, a small study of 21 patients who suffered a perioperative myocardial infarction who had undergone a coronary angiography prior to vascular surgery revealed that the majority of non-fatal perioperative myocardial infarctions occurred in arteries without high-grade stenoses, suggesting that these events may result from acute coronary artery thrombosis superimposed on fissured coronary artery plaques.52 Further, evidence to support this hypothesis comes from the Coronary Artery Revascularization Prophylaxis (CARP) trial53 which randomized 510 patients undergoing elective vascular surgery, who had at least one coronary artery with a ≥ 70% stenosis that was suitable for revascularization, to receive coronary artery revascularization or no coronary artery revascularization before vascular surgery. This trial did not demonstrate a significant reduction in periopera-tive myocardial infarctions in the patients who first underwent coronary revascularization prior to their non-cardiac surgery. If hemodynamically significant stenoses are the major cause of perioperative myocardial infarctions, it is surprising that there was no reduction in the risk of a peri-operative myocardial infarction despite coronary revascu-larization prior to non-cardiac surgery.
These limited and conflicting data regarding the patho-physiology of perioperative myocardial infarction highlight the need for large studies to provide insights. Such studies will inform the selection of appropriate preventive and management interventions to evaluate in subsequent large randomized controlled trials.
Diagnosing perioperative myocardial infarction
A recent review identified studies assessing patients undergoing non-cardiac surgery, that required patients to have at least one measurement of a cardiac biomarker or enzyme after surgery, and reported whether patients who suffered a perioperative myocardial infarction experienced cardiac symptoms.54 This review identified three studies that included a total of 1309 patients, and 38 of these patients suffered a perioperative myocardial infarction.55–57 Although the small sample size requires cautious interpretation, the findings were striking. Of the patients who suffered a perioperative myocardial infarction, only 14% experienced chest pain and only 53% had any potential sign or symptom that could have suggested a myocardial infarction.
These results are similar to the findings in the recent POISE trial, which also monitored cardiac biomarkers or enzymes in all patients for the first 3 days after surgery. Box 5.1 presents the definition used for myocardial infarction in the POISE trial, in which only 35% of the patients suffering a perioperative myocardial infarction had ischemic symptoms.5 The fact that approximately 75% of these myocardial infarctions occurred within the first 48 hours after surgery helps to explain why so many patients may not have experienced ischemic symptoms (i.e. this is a period when the majority of patients receive high-dose analgesic medication to blunt surgical discomfort). These data suggest perioperative myocardial infarctions present differently from the majority of myocardial infarction in the emergency room and that perioperative myocardial infarctions are at high risk of going undetected if troponin measurements are not monitored for the first few days after surgery. The ongoing VISION Study will provide further insights into this issue in a large unselected population of patients undergoing non-cardiac surgery who are having troponins monitored throughout the first 3 days after surgery.
BOX 5.1 Diagnostic criteria for perioperative myocardial infarction in the POISE trial
The diagnosis of perioperative myocardial infarction required any one of the following
| Criterion 1 | A typical rise of troponin or a typical fall of an elevated troponin or a rapid rise and fall of CK-MB. Patients also had to have one of the following: 1. ischemic signs (e.g. chest, arm or jaw discomfort; shortness of breath) 2. development of pathologic Q waves in two contiguous leads on an ECG 3. ECG changes indicative of ischemia in at least two contiguous leads 4. coronary artery intervention (e.g. percutaneous coronary intervention) 5. new or presumed new cardiac wall motion abnormality on echocardiography or new or presumed new fixed defect on radionuclide imaging |
| Criterion 2 | Pathologic findings of an acute myocardial infarction |
ECG, electrocardiogram.
Do perioperative myocardial infarctions matter and is surveillance justified?
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


