29
Equipment, Infection Control, and Safety
 Capital Equipment: Funding, Selection and Procurement
Capital Equipment: Funding, Selection and Procurement
Selection and procurement processes for capital medical equipment are complex and dynamic. Capital equipment is defined as “nonexpendable” and used to operate a business or provide a service. 1 Institutions have specific definitions in their policies for capital equipment. For example, any item costing more than $500 and/or with a useful life of more than 1 year may be considered capital. TEE systems cost more than $350,000, and prices vary with the choice of technology, probe configurations, and vendor relationships. TEE systems are hospital assets budgeted for and purchased from hospital capital funds, as opposed to being purchased by a physician group(s) within the institution. The rationale for this is that hospitals in the United States are reimbursed for capital expenditures through the federal government’s insurance program (i.e., Medicare) at rates set by the Centers for Medicare and Medicaid Services (CMS) payment systems. 2 The Medicare program consists of several parts; the hospital insurance part is known as Part A, and the supplemental medical insurance part paying for physician services is known as Part B. A portion of capital expenses are reimbursed to hospitals under Medicare Part A, while physician services are reimbursed under Medicare Part B.
• Clinical/technical capabilities
• Training and educational programs offered
• Information technology (IT) considerations
• Cost to purchase a TEE system
• Warranty, preventive maintenance, and repair terms
• Projection of all operating costs
• Are upgrades accomplished by updating software?
• Will additional equipment be necessary?
• What will be the associated costs?
• What are the durability and expected lifespan of the system?
As institutions move to electronic record keeping for all components of the patient’s medical record, one must evaluate the IT capabilities of the TEE equipment being considered. File management and image storage options are very important factors. TEE systems should meet digital imaging and communications in medicine (DICOM) specifications. DICOM is the standard for handling, storing, printing, and transmitting medical imaging information developed by the American College of Radiology (ACR) and the National Electrical Manufacturing Association (NEMA). 3 The capability to network wirelessly is also important. The hard drive of the TEE unit should have ample storage and user-friendly features for management of studies, including transfer to portable storage media.
 Evaluating Preventive Maintenance Programs and Product Support
Evaluating Preventive Maintenance Programs and Product Support
Quality of service and preventive maintenance costs, including TEE probe repair or replacement, are important considerations in selecting a vendor. Biomedical engineering and equipment managers are concerned with maintenance schedules and repairs. Expenses for maintenance and repair must be well understood because they are ongoing operational costs that must be forecast in the hospital’s operating budget. It is prudent to examine the pros and cons of preventive maintenance agreements. Vendors may offer one plan for maintenance of the ultrasound system and another for service for the TEE probes. As noted in James Carr’s white paper on TEE probe care, the majority of service calls generated for TEE systems are related to probe malfunctions because they are delicate and therefore easily damaged. 4 Service agreements for TEE probes may prove cost-effective and should be considered, particularly for large institutions with multiple systems. Vendors usually offer very attractive pricing for service agreements purchased at point of sale (POS). Replacement cost is significant and may exceed $50,000 per probe. For example, a multiple-year service agreement purchased at POS for a TEE probe may be offered at a fraction of the replacement cost. There is a high probability a probe will be damaged or require multiple repairs within the first 5 years of purchase, so this type of service agreement will minimize expenses associated with probe replacement.
Preventive maintenance programs for the ultrasound hardware should also be considered if the institution’s biomedical engineering department does not have the resources or expertise to perform planned or emergent maintenance. Replacement parts such as computer hardware, knobs, keyboards, and other components are generally included in maintenance agreements. Costs associated with preventive maintenance agreements are fixed costs. This is advantageous from a budgeting standpoint and will help minimize unexpected expenses.
 Additional Considerations
Additional Considerations
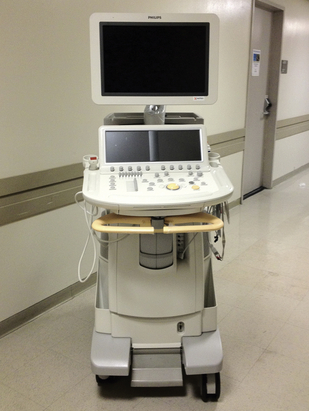
In addition to clinical capabilities and cost, there are other factors to consider that may influence selection of a particular system. Physical space in operating rooms and procedural areas is frequently limited. The footprint of a system and its portability may be important criteria in the selection process. As examples, Figures 29-1 through 29-3 depict TEE systems from three of the industry’s leading vendors: the Philips iE33 XMatrix, the Siemens ACUSON S2000, and G.E. Healthcare’s Vivid-e. Photo printers or DVD recorders are options physicians may request. A complete assessment of the user’s needs paired with the system’s capabilities will determine required accessories.

Figure 29-2 Siemens ACUSON S2000 System. (Reproduced with permission from Siemens Healthcare, Malvern, Pa.)
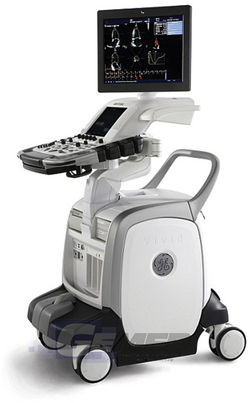
Figure 29-3 G.E. Healthcare’s Vivid-e System. (Reproduced with permission from G.E. Healthcare, Waukesha, Wis.)
 Care of the TEE System
Care of the TEE System
The TEE system requires regular maintenance and care to ensure minimal downtime and repairs. 5 Responsible parties include equipment managers and technicians, biomedical engineering personnel, and clinical users. Following the manufacturer’s recommendations for periodic maintenance and hospital equipment protocols for regular cleaning and probe care ensures minimal system downtime, extends the TEE’s lifespan, and ensures continued validity of equipment warranties. Use of cleaning solutions or lubricants not recommended by the manufacturer may damage probes and void the warranty. The clinician using the system will be the first to observe any abnormalities in ultrasound image quality, as well as structural damage to the system and TEE probe. Regular equipment checks should include:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree