Electrophysiology and Catheter-Ablative Techniques: Introduction
Since the 1980s, there have been dramatic advances in clinical electrophysiology procedures to diagnose and treat cardiac arrhythmias with catheter ablation. Electrophysiologic studies (EPS) generally refer to the catheterization procedure that provides diagnostic information. An EPS is often combined with catheter ablation to treat the identified arrhythmia.
Diagnostic Electrophysiologic Studies
Diagnostic EPS are performed to diagnose an arrhythmia when the diagnosis has not been obtained with noninvasive techniques or as part of an ablation procedure to treat the arrhythmia. EPS can be performed when an arrhythmia is suspected, but symptoms are intermittent and electrocardiographic (ECG) monitoring has not captured the symptomatic rhythm or there is concern that the arrhythmia may be life threatening, as in a patient with structural heart disease and syncope. Diagnostic EPS provides information about cardiac automaticity and conduction (sinus node, atrioventricular [AV] node, and His-Purkinje function) as well as inducible arrhythmias. Programmed electrical stimulation is performed to attempt to induce suspected or documented arrhythmias. EPS can help to detect arrhythmia causes of syncope and distinguish ventricular tachycardia (VT) from supraventricular tachycardia (SVC) with aberrancy (see Fig. 44–2C) and has a role in assessing the risk of ventricular arrhythmias in patients with known or suspected diseases associated with a risk of sudden death. Common uses of EPS are summarized in Table 44–1.
| Suspected or documented bradyarrhythmias |
Sinus node function
|
AV node function
|
His-Purkinje system conduction
|
| Suspected or documented tachycardias: inducible arrhythmias |
Paroxysmal supraventricular tachycardias
|
Ventricular tachycardias
|
Uses of diagnostic electrophysiologic studies
|
Specific protocols for EPS vary among laboratories. Commonly femoral venous access is obtained to insert three electrode catheters positioned at various areas in the heart: one in the right atrium, one in the right ventricular (RV) apex, and one at the His bundle location (Fig. 44–1). For evaluation of SVCs, a coronary sinus catheter is often added to record left atrial activation (Fig. 44–2). Baseline conduction intervals (AH and HV) are determined. In adults, the HV interval has a narrow acceptable normal range (35-55 ms). If this interval is shorter than 35 ms, an accessory pathway is usually present producing ventricular preexcitation during sinus rhythm, suggesting a possible mechanism for SVC (Wolff-Parkinson White syndrome). A prolonged HV interval indicates disease of the distal conduction system. In patients with syncope, an HV interval longer than 70 ms suggests possible AV block as a cause, warranting consideration of permanent pacing. An HV interval longer than 100 ms is a severe abnormality that warrants placement of a permanent pacemaker even absent a syncopal spell.1
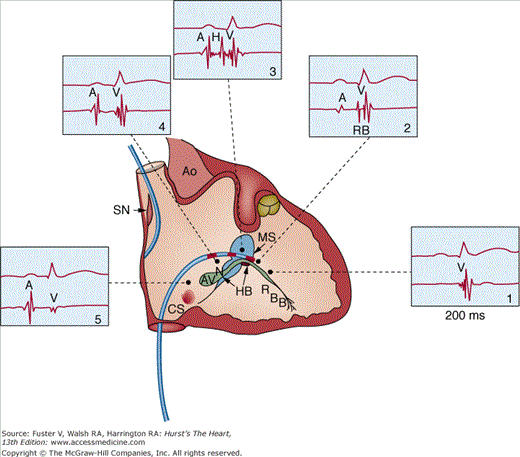
Figure 44–1
Intracardiac recordings from the specialized conduction system in the atrioventricular (AV) junction. The recording of various electrograms along the right side of the interventricular septum with gradual withdrawal of the catheter across the tricuspid valve is shown. The intracardiac recordings are labeled. Numbers 1 through 5 refer to intracardiac location of catheters along with corresponding electrogram. A, atrial deflection; Ao, aorta; AVN, atrioventricular node; CS, coronary sinus; H and RB, His and right bundle potentials; HB, His bundle; MS, membranous septum; RBB, right bundle branch; SN, sinus node; V, ventricular deflection. From Gallagher JJ, Damato AN. Technique of recording His bundle activity in man. In: Grossman W, ed. Cardiac Catheterization and Angiography. Philadelphia, PA: Lea & Febiger; 1980:283. Reproduced with permission from the publisher and authors.
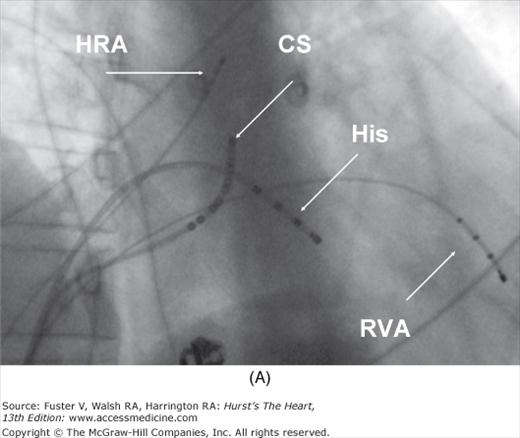
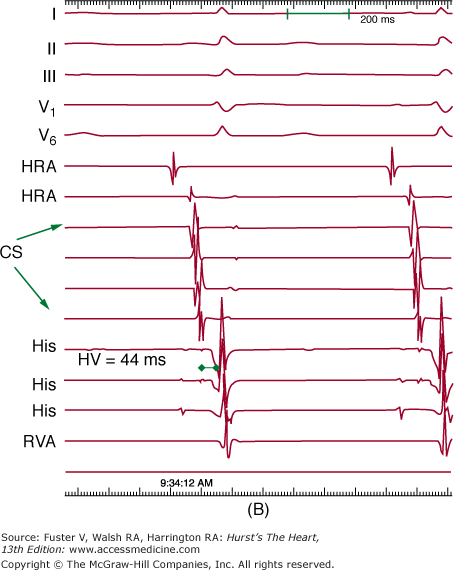
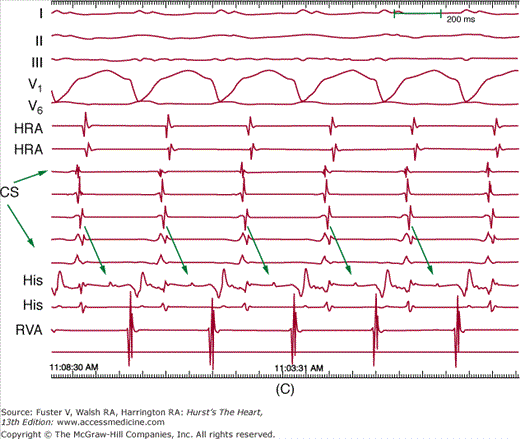
Figure 44–2
Setup for electrophysiology study and supraventricular tachycardia ablation. A. A right anterior oblique fluoroscopic image of a typical initial catheter setup. Catheters are placed via the femoral vein. There is a quadripolar catheter in the high right atrium (HRA) and in the right ventricular apex (RVA). There is another quadripolar catheter positioned to measure a His bundle (His) electrogram and a decapolar catheter in the coronary sinus (CS). B. Baseline conduction intervals in sinus rhythm with a normal HV interval of 44 ms. Note that atrial activation times with the P wave on the surface electrocardiogram (ECG) and starts with the HRA catheter that is nearest the sinus node. C. A patient in atrioventricular reentrant tachycardia with left bundle-branch block. Note that on the surface leads, there is a wide complex with QS pattern in V1. Arrows indicate the HB deflection preceding each QRS complex, indicating conduction down the His-Purkinje system producing the observed wide complex on the surface ECG.
Sinus node function can be assessed by atrial pacing. The sinus node recovery time is determined by pacing faster than the sinus rate to overdrive-suppress the sinus node. The interval between the last paced beat and the sinus beat that returns after pacing is the sinus node recovery time. The sinus node is well innervated and responsive to autonomic tone. Sympathetic stimulation and vagal withdrawal can compensate for abnormal sinus node function. EPS does not reliably detect abnormal sinus node function. Unfortunately, although these measurements can be helpful when they are abnormal, they do not rule out intermittent bradyarrhythmias from sinus node dysfunction. A prolonged AH interval (>120 ms) indicates abnormal AV node function. AV block within the AV node during atrial pacing at a rate slower than 100 to 120 beats/min also indicates abnormal AV nodal conduction but can be normal when vagal tone is high. Sympathetic tone can compensate for abnormal AV node function. Thus, EPS is limited in its ability to detect sinus and AV node dysfunction even when it is clinically important and symptomatic. Long-term ambulatory monitoring, which can be accomplished with implantable recorders, is more likely to detect intermittent bradyarrhythmias caused by sinus or AV node dysfunction when these arrhythmias produce infrequent symptoms.2
Programmed electrical stimulation of the atrium and ventricles is used to attempt to induce tachyarrhythmias. Stimulation protocols usually pace at a regular rate for eight or more beats and then deliver a premature extrastimulus, mimicking atrial or ventricular premature beats in progressively earlier intervals. Multiple extrastimuli may be used. Increasing the number of extrastimuli improves the likelihood of initiating diagnostic arrhythmias but also increases the likelihood of initiating nonspecific arrhythmias that may not be related to the symptoms or indicative of risk.
Pacing from different atrial or ventricular sites as well as manipulation of autonomic tone with isoproterenol or atropine may be used to facilitate arrhythmia initiation. When a tachycardia is initiated, the mechanism can be determined from analysis of activation recorded from the multiple catheters and pacing maneuvers during the tachycardia. SVC with aberrancy can be reliably distinguished from VT by assessment of the relation of His bundle activation to the QRS during an induced tachycardia (see Fig. 44–2C). In addition, recording from the atrium during the tachycardia allows reliable detection of AV dissociation when present during VT when it may not be evident from the surface ECG.
In patients who have not had symptoms of an arrhythmia but who are at risk for sudden death because of the presence of underlying heart disease, EPS has been used to detect the presence of inducible arrhythmias that indicate an increased risk that an arrhythmia may spontaneously occur, particularly if VT causing sudden death is a concern. Inducible sustained monomorphic VT or polymorphic VT is associated with an increased risk of sudden death in patients who have depressed ventricular function from a prior myocardial infarction and often warrants placement of an implantable defibrillator (ICD) (see Chap. 46). EPS has not been useful for risk stratification in patients with nonischemic dilated or hypertrophic cardiomyopathies. EPS has also been used to assess inducible ventricular arrhythmias in patients with suspected Brugada syndrome and arrhythmogenic RV dysplasia (see Chap. 82).
The risks of EPS are related to the catheterization procedure and are similar to those of left or right heart catheterization. Vascular access complications and femoral hematomas are the most common complications. Complications related to sedation may occur, but serious complications are rare. Cardiac perforation with tamponade, deep venous thrombus, and pulmonary embolism may occur.
Catheter Ablation
Catheter ablation requires making an accurate diagnosis of the arrhythmia, mapping to determine the precise location of the tissue causing the arrhythmia, placement of the ablation catheter on this site, and effective ablation of the tissue. The success of the procedure varies with the type of arrhythmia and its cardiac origin. Catheter positioning for mapping is assisted by fluoroscopy (see Fig.44–2) and often by sophisticated mapping systems that display the location of the catheters and a three-dimensional representation of the anatomy as well as the electrophysiologic characteristics of the tissue. Many mapping systems display continuous catheter position without the need for fluoroscopy, reducing radiation exposure. Integration of cardiac images from magnetic resonance imaging (MRI) or computed tomography (CT) and incorporation of real-time echocardiographic data are commonly used when extensive ablation is required over complex anatomy, as for the extensive left atrial ablation required for atrial fibrillation (AF).
Tissue damage for ablation is most commonly achieved with application of radiofrequency (RF) energy, which creates a burn by resistively heating the tissue. A cryocatheter that freezes tissue is also available. Acoustic and laser-based ablation systems are also being evaluated. The ablation lesion heals with replacement of myocardium by fibrosis. Ablation lesions can be “incomplete” such that initial edema resolves and the tissue recovers, resulting in arrhythmia recurrence.
Procedure risks include those for EPS (above) but vary with the particular type of ablation. Procedures that require marked anticoagulation, such as ablation for AF, can be expected to have greater risk of femoral hematomas and tamponade if cardiac perforation occurs. Arrhythmias that require left atrial or ventricular mapping and ablation have a risk of systemic embolization. For many arrhythmias, the risks are sufficiently low that catheter ablation has become an important and common therapy
Supraventricular Tachycardias
AV nodal reentrant tachycardia (AVNRT) is the most common regular paroxysmal SVC, representing a significant portion of all cases referred for catheter ablation of regular SVC. Women are more commonly affected than men. Although episodes are not life threatening, symptoms of palpitations, presyncope, and syncope can interfere with daily activities and exercise. Catheter ablation is recommended when episodes are poorly tolerated or fail to respond to pharmacologic therapy and is a reasonable option for patients who desire complete control of arrhythmia without medications.3
During EPS, evidence of dual pathways for conduction with a discontinuity in conduction times through the AV node is usually evident. Administration of isoproterenol during programmed electrical stimulation is often required to induce the tachycardia. AVNRT typically has a 1:1 relationship of V to A, often with the A occurring synchronously with the QRS or shortly after the QRS because the slow pathway is used anterogradely, and the fast pathway portion of the AV node forms the retrograde limb of the circuit. When the circuit revolves in the reverse direction, an atypical form with a long RP interval occurs. The mechanism is confirmed by pacing maneuvers during tachycardia.
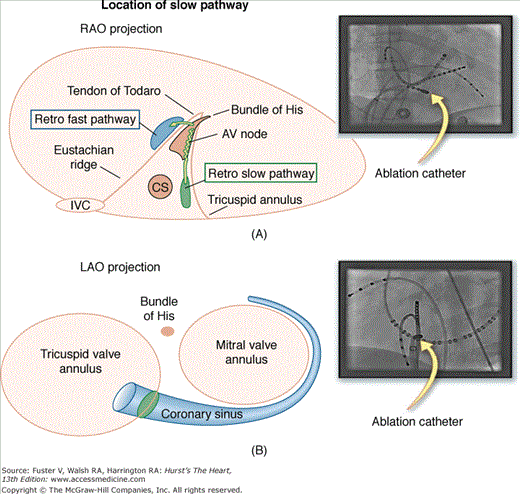
Catheter ablation is feasible because of the AV nodal structure, which consists of a compact portion that joins the His bundle and two or three lobes that extend out from the compact node. In patients with AVNRT, the lobe that extends along the tricuspid annulus toward the coronary sinus forms a pathway for slow conduction between the os of the coronary sinus and the septal leaflet of the tricuspid valve (Fig. 44–3) that can be ablated without damaging conduction through the compact portion of the AV node, thereby leaving AV conduction intact.4 This anatomic AV nodal slow pathway is targeted for ablation (see Chap. 37). Success is achieved in more than 95% of patients. The major risk is inadvertent damage to the compact AV node producing heart block that requires permanent pacemaker implantation in approximately 0.8% of patients.5 Use of cryoablation may be associated with a lower risk of heart block but also with a somewhat lower long- term success rate.6 A tendency to sinus tachycardia may occur after successful slow pathway ablation, lasting for up to 6 months, possibly related to interruption of vagal fibers in the inferoseptal region.7
Figure 44–3
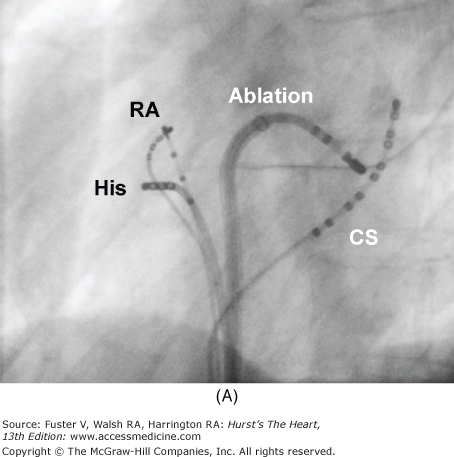
Atrioventricular (AV) nodal reentry tachycardia. Illustrations with correlated fluoroscopic images of the right anterior oblique (RAO; A) and left anterior oblique (LAO; B) projects. The ablation catheter is placed in the region of the coronary sinus (CS) ostium. This is the location of the slow pathway input to the AV node. Ablation at this site transects the AV node reentry circuit and is highly successful (success rates >95%) in treating typical AV node reentry. The compact AV node is located close to the annulus near the septal leaflet of the tricuspid valve. Because of the complexity of the right atrium, the atrial connections to the AV node may be discrete in some patients, forming the fast and slow pathways—the limbs of the AV node reentry circuit. IVC, inferior vena cava.
AV reciprocating tachycardia (AVRT) using an AV accessory pathway is the second most common paroxysmal SVC and has a slight male predominance. When the accessory pathway manifests antegrade conduction from atrium to ventricle, preexcitation is usually evident during sinus rhythm because of depolarization of a portion of the ventricle from conduction over the accessory pathway. A short PR interval and slurred delta wave on the ECG are hallmarks of anterograde ventricular preexcitation. Antegrade conduction over an accessory pathway also indicates a potential risk of a very rapid ventricular response to AF that can cause ventricular fibrillation (VF; see later), or tachycardias with activation over the accessory pathway that have a wide QRS that can mimic VT. Pathways that conduct only from ventricle to atrium are termed “concealed” because preexcitation is absent during sinus rhythm, but AVRT still can occur. The usual circuit causing tachycardia is caused by antegrade conduction over the AV node, producing a narrow QRS (or typical right or left bundle-branch aberrancy) and retrograde conduction, from ventricle to atrium, over the accessory pathway.8
During EPS, ventricular pacing often shows conduction from ventricle to atrium over the pathway, rather than the AV node. EPS typically initiates the arrhythmia, allowing the mechanism to be confirmed from additional pacing maneuvers and analysis. Catheter ablation is the standard treatment for patients with symptomatic Wolff-Parkinson-White (WPW) syndrome with preexcitation, for AVRT caused by a concealed accessory pathway that fails antiarrhythmic drug therapy, and when drug therapy is not desired.3 Accessory AV pathways occur anywhere along the tricuspid or mitral annulus or in the septum. The majority traverse the mitral annulus. These pathways can be approached retrogradely by inserting a steerable electrode catheter into the femoral artery through the aortic valve and then to the mitral annulus or by a transseptal approach through the fossa ovalis to the left atrium (Fig. 44–4A).9 Many septal and all right free wall pathways are accessible by a venous approach to the right heart.
Figure 44–4
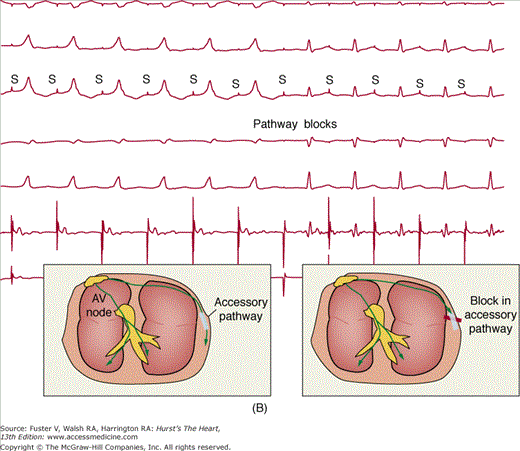
A. Ablation of a left-sided accessory pathway in a patient with Wolff-Parkinson-White syndrome. Shown is a left anterior oblique fluoroscopic image depicting catheter position for transablation of a left-sided accessory pathway by a transeptal approach. A decapolar catheter is seen in the coronary sinus (CS). The ablation catheter (Ablation) is passed through a sheath, which crosses the fossa ovalis into the left atrium for mapping along the tricuspid valve annulus. B. Five surface electrocardiographic leads and two intracardiac leads depicting ablation of a left lateral accessory pathway during atrial pacing. The right atrium is being paced (pacing stimuli indicated by S). Pacing stimuli initially conduct from the atrium to the ventricles over the atrioventricular (AV) node and an accessory pathway producing a pattern of ventricular preexcitation with a wide QRS with a slurred initial delta wave as indicated by the left-hand schematic at the bottom of the figure. The pathway blocks within seconds of the application of radiofrequency energy with sudden narrowing of the QRS complex (Pathway blocks) indicating that conduction to the ventricles is occurring only over the normal conduction system of AV node and His bundle (right-hand schematic at the bottom of the figure). RA, right atrium.
Recent multicenter catheter ablation results, including a large, prospective, voluntary registry, show overall success rates of 95% (Fig. 44-4B), with a risk of recurrent pathway conduction caused by healing of the ablation lesions of 3% to 10%.3,10 Serious complications related to left or right heart catheterization occur but are uncommon. Heart block can occur with attempted ablation of pathways that are located close to the AV node.
Approximately 25% of patients with overt WPW syndrome have pathways that place them at risk of VF if AF occurs. Consequently, EPS with curative catheter ablation is generally recommended for patients with symptomatic arrhythmias and WPW syndrome. The management of asymptomatic patients who are found to have preexcitation on ECG is controversial.11,12 The risk of sudden death in these patients is estimated to be one in 1000 per year, similar to the risk of the EPS procedure. EPS can be considered to assess whether the pathway is capable of dangerously rapid conduction. Individualized consideration of the risks and benefits is warranted.
Focal atrial tachycardia (AT) represents approximately 10% of narrow complex tachycardias referred for ablation. These tachycardias can be paroxysmal or incessant or may occur in repetitive bursts. They may respond to β-blockers, calcium channel blockers (CCBs), and membrane active antiarrhythmic drugs but can also be ablated.13,
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree