Effects of Positive-Pressure Ventilation on the Pulmonary System
Learning Objectives
On completion of this chapter, the reader will be able to do the following:
1 Recognize the presence of barotrauma or extra-alveolar air based on patient assessment.
2 Recommend an appropriate intervention in patients with barotrauma.
6 Compare the clinical findings associated with hyperventilation and hypoventilation.
7 Recommend ventilator settings in patients with hyperventilation and hypoventilation.
8 Identify a patient with air trapping.
9 Provide strategies to reduce auto-PEEP.
10 Suggest methods to reduce the work of breathing (WOB) during mechanical ventilation.
11 List the possible responses to an increase in mean airway pressure in a ventilated patient.
Key Terms
• Asynchrony
• Barotrauma
• Cardiac tamponade
• Chest-abdominal paradox
• Deep sulcus sign (chest radiograph)
• Hyperinflation
• Hyperventilation
• Hypoventilation
• Multiple organ failure
• Multisystem organ failure (multiple-organ dysfunction syndrome)
• Overdistention
• Perivascular
• Volutrauma
There are a number of inherent risks and complications associated with the use of mechanical ventilators. These include ventilator-associated and ventilator-induced lung injury, the effects of positive-pressure ventilation (PPV) on gas distribution and pulmonary blood flow, hypoventilation and hyperventilation, air trapping, oxygen toxicity, increased WOB, patient-ventilator asynchrony, mechanical problems, and complications of the artificial airway. This chapter reviews the cause and adverse effects of these complications.
Lung Injury With Mechanical Ventilation
During the 1970s and 1980s it was not uncommon for patients to be ventilated with pressures greater than 45 cm H2O. Indeed, nearly 20% of patients diagnosed with ARDS were, at some point in their management, ventilated with pressures of 80 cm H2O or greater and volumes in the range of 10 to 12 mL/kg.1 This is interesting, considering that it has been known for more than three decades that using these high levels of pressure and volume can cause lung injury, referred to as barotrauma or volutrauma.
Barotrauma implies trauma that results from using high pressures. Volutrauma implies damage from high distending volumes rather than high pressures. Evidence suggests that high distending volumes result in overdistention and lung injury, whereas high distending pressures alone do not cause lung injury. Overdistention causes the release of inflammatory mediators from the lungs that can lead to multiorgan failure. This latter response has been termed biotrauma.
Repeated opening and closing of lung units, also called recruitment/derecruitment, generates shear stress, which results in direct tissue injury at the alveolar and pulmonary capillary level, as well as the loss of surfactant from these unstable lung units. Shear stress injury and loss of surfactant have been termed atelectrauma. The following section provides a summary of these various aspects of lung injury as they relate to mechanical ventilation.
Ventilator-Associated Lung Injury Versus Ventilator-Induced Lung Injury
The terms ventilator-associated lung injury (VALI) and ventilator-induced lung injury (VILI) have been used frequently in the literature with some inconsistency regarding their meaning. The term VALI is generally used when referring to lung injury occurring in humans that has been identified as a consequence of mechanical ventilation.2 The most common forms of VALI include ventilator-associated pneumonia (VAP), air trapping, patient-ventilator asynchrony, and extra-alveolar gas (barotrauma) such as pneumothorax and pneumomediastinum. (See Chapter 14 for a discussion of ventilator-associated pneumonia.)
VILI is lung injury that occurs at the level of the acinus. It is the microscopic level of injury that includes biotrauma, shear stress, and surfactant depletion (atelectrauma). VILI can be specifically studied only in animal models because ventilator strategies that will potentially harm the lung cannot be performed on human subjects during research investigations.
VILI is a form of lung injury that resembles ARDS. It has been studied using animal models and apparently occurs in patients receiving inappropriate mechanical ventilation. VILI is difficult to identify in humans because its appearance is based on radiologic and clinical findings, which overlap with the findings that occur with the underlying pulmonary pathology, such as ARDS. In fact, it is reasonable to assume that acute lung injury (ALI) and ARDS may be partially the result of ventilator management rather than the progression of the disease.3 This supports the idea that mechanical ventilation not only saves lives but has the potential to worsen preexistent lung injury.1
The following section defines and describes the various forms of VALI and VILI (Key Point 17-1).
Barotrauma or Extra-Alveolar Air
As mentioned, it has been known for some time that PPV increases the risk of barotrauma. This type of injury involves the formation of extra-alveolar gas, such as subcutaneous emphysema, pneumothorax, pneumomediastinum, pneumoperitoneum, and pneumopericardium.
The risk of rupture to the lung is greater for patients with lung bullae or chest wall injury. A number of conditions can predispose a patient to barotrauma (extra-alveolar air). Some of these include the following4,5:
• High peak airway pressures with low end-expiratory pressures
• Bullous lung disease such as may occur with emphysema or a history of tuberculosis
• High levels of PEEP with high tidal volumes (VT)
• ALI/ARDS
Barotrauma occurs when gas under pressure causes alveolar rupture. Air is forced into the interstitium of an adjacent bronchovascular (perivascular) sheath in the area of the distal noncartilaginous airways.4,6 The “escaped” air moves along the sheath toward the hilum and mediastinum, causing a pneumomediastinum (Fig. 17-1).4,7 Air can then break through the pleural surface of the mediastinum into the intrapleural space, resulting in a pneumothorax. Pneumothorax may be unilateral or bilateral. Air in the mediastinum also may dissect along tissue planes, producing subcutaneous emphysema. Pneumoperitoneum may follow pneumomediastinum and occurs when air dissects initially into the retroperitoneum. Air that is trapped under the diaphragm in the peritoneum may interfere with effective ventilation.
From its location in the mediastinum, air can also dissect along tissue planes near the heart and form a pneumopericardium.2 The escaped air can be reabsorbed into adjacent tissues and resolve itself. If it is not reabsorbed by the body, evacuation by a drainage system may be required. Failure to remove this extra-alveolar air can lead to life-threatening problems, such as tension pneumothorax or pneumopericardium.
Subcutaneous Emphysema
Detection of subcutaneous emphysema is not difficult. It may be visible as a puffing of the skin in the patient’s neck, face, or chest and may even be present in distal areas such as the feet and abdomen. The skin feels crepitant to the touch. Subcutaneous emphysema usually occurs without complication and tends to clear without treatment as mean airway pressures are reduced. However, if it is present with dyspnea, cyanosis, and increased peak pressures, it may be accompanied by a pneumothorax.
Pneumomediastinum
Pneumomediastinum can lead to compression of the esophagus, great vessels, and the heart. It usually can be easily identified on chest radiographs. Treatment depends on the severity of the problem and its effect on adjacent structures. In severe cases, pneumomediatinum can cause cardiac tamponade. If the air is not removed, cardiac tamponade can ultimately lead to cardiopulmonary arrest.
Pneumothorax
Early clinical studies suggested that the most common clinical manifestation of extra-alveolar air was pneumothorax.8,9 Although studies have shown that the incidence of barotrauma is relatively low (2.9%),10 results vary from study to study. Interestingly, the reduced incidence of barotrauma may be associated with use of lower VT and lower airway pressures.
Pneumothorax may lead to lung collapse, with mediastinal shifting occurring away from the affected side. Pneumothorax also can be detected by a resonant or hyperresonant percussion note and absence of breath sounds on the affected side, and chest radiographs will indicate lack of vascular markings on the affected side. Treatment usually requires thoracotomy and placement of a chest tube. Because pleural air rises to the highest (nondependent) area of the thorax, the affected area will depend on the patient’s position. In the supine patient this is an area over the anterior surface of the lung. This can make detection of a small pneumothorax difficult when evaluating a chest radiograph taken with the patient supine (Case Study 17-1).
Another way of detecting a pneumothorax in patients on mechanical ventilation is to observe progressive changes in peak pressure. Increases in peak pressure occurring within a short period, such as a few minutes to a few hours, may signal the presence of pneumothorax of either rapid onset or one caused by a slow, insidious leak. Physical examination and a chest radiograph should be used to confirm the diagnosis.
Because a simple pneumothorax can develop into a tension pneumothorax, careful monitoring is essential. Administering excessive amounts of positive pressure may aggravate the presence of air in the pleural space, so manual ventilation with a resuscita-tion bag on 100% oxygen may be advisable until the problem can be treated.7 It is important, however, for the clinician to avoid using excessive pressure with manual compression of a resuscitation bag.11
A tension pneumothorax is a life-threatening situation that must be treated immediately. A tension pneumothorax occurs when air enters the pleural space and becomes trapped. Pressure gradually builds, collapsing the affected lung. Mediastinal structures will shift in the thorax away from the area of tension and put pressure on the heart and the unaffected lung. Tracheal deviation and neck vein distention are possible signs. Breath sounds will be absent and the percussion note tympanic. A chest radiograph on a patient with a tension pneumothorax is not advisable because it might delay life-saving treatment. In a chest radiograph of a tension pneumothorax, one diaphragm will be more depressed than the other and may display a deep sulcus sign, with air appearing adjacent to the depressed diaphragm.
Treatment for tension pneumothorax involves inserting a 14-gauge needle, or similar device, into the anterior second to third intercostal space on the affected side in the midclavicular line over the top of the rib with the patient in the upright position. This maneuver can be life saving. While waiting for trained personnel to be summoned to perform this procedure, the respiratory therapist should decrease mean airway pressures as much as possible while using manual ventilation with a high fraction of inspired oxygen (FIO2).
Pneumoperitoneum
Pneumoperitoneum generally follows pneumomediastinum. It occurs when air dissects into the retroperitoneal space. The peritoneum can also rupture, resulting in air moving into the peritoneal cavity. As you might expect, this can be very painful. If a significant amount of air is present, it can interfere with the movement of the diaphragm and reduce effective ventilation.
Barotrauma or Volutrauma
In early studies, researchers tried to determine whether lung injury was caused by high pressures (barotrauma) or high volumes (volutrauma). Dreyfuss and colleagues coined the term volutrauma to describe the injurious effects of mechanical ventilation they observed in laboratory studies using an animal model.12 These investigators found that it was not high pressure but the relatively large regional volumes that overstretched compliant areas of the lung that resulted in alveolar stretch and edema formation in these areas.12,13
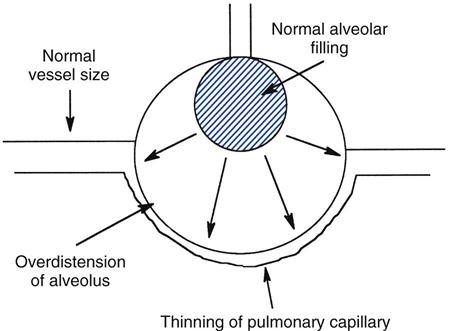
It is now generally accepted that using inordinately high tidal volume can lead to lung overdistention and iatrogenic lung injury. Overdistention occurs in those areas of the lungs where high distending pressures—in other words, high transpulmonary pressures (alveolar pressure-pleural pressure [Palv−Ppl])—are present. Indeed, pressures as low as 30 to 35 cm H2O have been shown to cause lung injury in animals.4,12
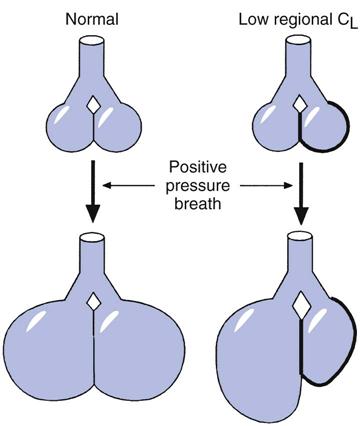
Because regional differences in lung compliance and transpulmonary pressures (PL) occur in most pulmonary disorders, positive pressure applied to the lung tends to produce larger volumes in more compliant lung areas (Box 17-1). The resulting overdistention to these areas causes acute alveolar injury and the formation of pulmonary edema by both increased permeability and filtration mechanisms. Tidal volumes of 10 to 12 mL/kg can cause overdistention of these areas of greater compliance.
Additional animal studies found that when the chest wall movement was restricted by binding the thorax and pressure was applied to the lungs, less lung injury occurred.12–14 Thoracic binding prevented severe transpulmonary (alveolar-distending) pressure. Furthermore, alveolar stretch and edema formation did not occur under these conditions. In the clinical setting, restriction to chest wall movement is present when patients are in the prone position, in severely obese patients, or when heavy dressings are used to manage surgical sites of chest or chest wall injuries (Box 17-2).15
To understand the importance of pressure in this setting and its distribution, several circumstances that affect lung pressures must be examined. Pressure at the upper airway is not equal to alveolar pressure (Palv) except when flow is zero and the airway is open. (This is usually termed plateau pressure, or Pplateau.) To interpret Palv, or Pplateau, the circumstances in which it is measured should be known. The following are seven of these circumstances15:
4 The lungs are normal, but the abdomen is turgidly overdistended (similar to the first circumstance).
5 The lungs are very stiff, leaving pleural pressure near normal (e.g., 5 cm H2O).
7 Both lungs are dangerously overdistended inside a normal chest wall.
In the first four examples, structures around the lung (e.g., chest wall and abdomen) oppose most of the alveolar pressure; the pleural pressure is high, but the distending pressure is within safe limits. Only the last three examples are situations in which lung distending pressure (i.e., the transpulmonary pressure, or Palv − Ppl) is abnormally high and thus can cause lung injury. Palv can be high by itself without causing lung damage, but if Palv − Ppl is high, lung damage is more likely to occur.
Lung injury from overdistention is much more subtle than air leaks described in the preceding section on barotrauma. Overdistention lung injury causes excessive stretching of alveolar cells, the formation of edema, and the release of inflammatory mediators, also called chemical mediators. As mentioned earlier, the release of these chemical mediators is termed biotrauma.
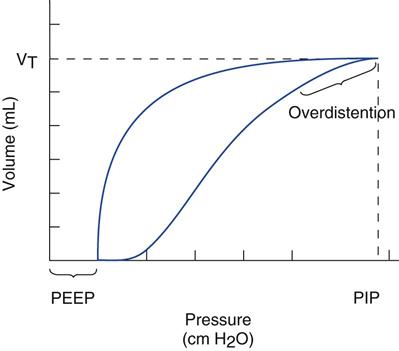
Figure 17-2 shows a pressure-volume curve that indicates the presence of overdistention. The shape of this curve is sometimes said to have a “duck-billed” appearance. Most clinicians now believe that this portion of the curve occurs with overdistention of more compliant areas of the lung, resulting in volutrauma. For the sake of simplicity, the term barotrauma will be used in this text to imply the leaking of air into body tissues (extra-alveolar air leak) and the term volutrauma to describe damage from overdistention that occurs at the alveolar level and involves alveolar and interstitial edema formation, alveolar stretch, and biotrauma.
Atelectrauma
The term atelectrauma is used to describe the injuries to the lungs that occur because of repeated opening and closing of lung units at lower lung volumes. Atelectrauma can occur in the management of ALI/ARDS when low tidal volumes are used and inadequate levels of PEEP are applied (see Chapter 13). Under these circumstances, alveoli tend to open on inspiration and close on expiration. (This most often occurs in the dependent areas of the lung. In supine patients this would be the dorsal area near the spine.) The repeated opening and closing of lung units in ALI/ARDS produces three primary types of lung injury: shear stress, alteration and washout of surfactant, and microvascular injury.16–18
Research studies involving animal models showed that ventilating pressures of 30 to 80 cm H2O produce atelectrauma with resulting reduced compliance and severe hypoxemia. Atelectrauma may be described as alveolar rupture, interstitial emphysema, or perivascular and alveolar hemorrhage, which can eventually lead to death.12,19–26 Death occurred in experimental animal models in some cases within an hour.
Shear Stress
Shear stress occurs when an alveolus that is normally expanded is adjacent to one that is collapsed (atelectasis) and unstable. As airway pressure increases during inspiration, the normal alveolus inflates, but the collapsed unit does not. In the interstitial space between the two, force is exerted as these two units move or slide against each other. There is a potential zone of risk at the interface of open and closed lung units. This is similar to what occurs when a paper clip is repeatedly twisted; eventually the paper clip breaks. In the lung, the stress pulls normal tissues apart, resulting in physical damage to the alveolar cells, particularly epithelial and endothelial cells (pulmonary microvasculature). The term shear stress has been applied to this type of situation. The amount of stress across the entire lung can be estimated by using transpulmonary pressure (Pplateau−Ppl), where Pplateau represents Palv and Ppl is the intrapleural pressure (Fig. 17-3, Key Point 17-2).5,27
The importance of shear stress has been known for a number of years. In fact, more than 30 years ago, Mead and colleagues28 calculated from a model that a transpulmonary pressure of only 30 cm H2O could result in a stress of 140 cm H2O being exerted between two adjacent alveoli as one expands and the other unstable unit remains collapsed. Not surprisingly, this force acting on the delicate tissues of the acinus can result in tearing of alveolar epithelium and capillary endothelium along with other structural injury.
Surfactant Alteration
A second consequence of the repeated opening and closing of alveoli involves reorientation of the surfactant molecules lining the alveolar surface. In the alveolus, surfactant forms a molecular layer between the air and the liquid alveolar surface. During alveolar collapse, as the surface area of the alveolus decreases, the surfactant molecules can form together until some actually pop out or get squeezed out at low lung volumes. These “used” lipids do not rapidly spread as the alveolus reopens.2 Rather, it is theorized that newly secreted surfactant replaces surfactant that is lost from the affected area. The greater the decrease in surface area during exhalation (i.e., the more the alveolar volume changes), the greater the number of molecules likely to migrate from the affected area. Thus, a greater amount of new surfactant is required to stabilize the lung unit.29 How quickly and for what length of time the alveolar cells can continue to produce an adequate amount of surfactant are uncertain. It is believed that eventually not enough surfactant will be present and the alveolus will become very unstable. Besides the effects of opening and closing of alveoli on surfactant production, it has been suggested that overdistention also reduces surface tension and is believed to alter surfactant function.5
Biotrauma
Mechanical stress disrupts normal cell function, strains normal cell configuration, and can also lead to an inflammatory response in the lungs.13 Current theory suggest that pulmonary cells, particularly epithelial cells, become distorted during mechanical ventilation when they are overstretched (overdistention). This overdistention causes the release of chemical mediators (i.e., cytokines). In addition to epithelial cells, the alveolar macrophages are another important source of inflammatory mediators, which are produced in response to a stretching strain and result in a potential molecular and cellular basis for VILI (Box 17-3).30–35
It is important to understand that ALI/ARDS does not have to be present for this inflammatory response to occur. However, when the inflammatory mediators are released, the lung begins to resemble that of a lung with ALI/ARDS. Indeed, the damage that can be caused by ventilator mismanagement may actually be indistinguishable from the underlying disease process of ARDS.3
Multiple-Organ Dysfunction Syndrome
Chemical mediators produced in the lung can leak into the pulmonary blood vessels. The circulation then carries these substances to other areas of the body and sets up an inflammatory reaction in other organs, such as the kidneys, gut, and liver.31,36 The release of mediators may therefore lead to multiple organ failure, also called multisystem organ failure and multiple-organ dysfunction syndrome.2,37,38
Treating patients with ARDS with lung-protective strategies, such as low VT and therapeutic PEEP, can significantly reduce morbidity and mortality rates in these patients (see Chapter 13).35,39–41 It also has been suggested that hypercapnia may be beneficial in patients with ARDS who are difficult to ventilate because it has an antioxidant effect and may actually reduce inflammation. Therapeutic hypercapnia may actually be a more appropriate name, but additional studies are needed (see Chapter 13).13,42–44
Vascular Endothelial Injury
A third problem that can occur with repeated alveolar collapse and reopening involves the pulmonary microvasculature. Recall that during a positive-pressure breath, alveolar capillaries flatten out, but corner microalveolar vessels open wider (see Fig. 13-16). The interstitial areas adjacent to the corner vessels develop negative pressure relative to the inside of the vessels. This negative-pressure gradient tends to pull fluid and blood products out of the vessels and into the space. Thus the alveoli and perivascular areas become edematous.
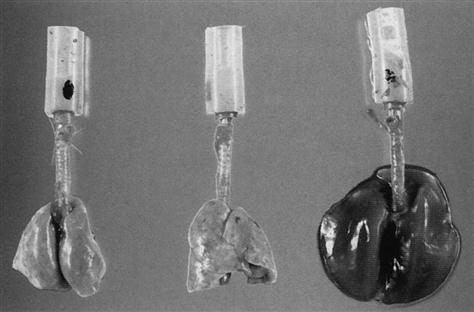
If the vascular pressure of the lung is further increased, at a certain point the vessel can rupture and release red blood cells and other blood components into the alveoli and interstitial space (Fig. 17-4). In Mead’s model, a stress of 140 cm H2O was proposed as occurring between two alveoli as one expanded and the other unit remained collapsed.28 This pressure could also be transmitted to the pulmonary vessels, which could represent a second cause of vessel rupture. The increased fluid leaking into the lung would create a dramatic increase in lung weight, which may be one of the mechanisms associated with the hemorrhagic appearance of lungs on autopsy in animal models subjected to low VT ventilation without PEEP (Fig. 17-5).45 Studies using a canine model have shown that it takes 90 to 100 mm Hg to produce this phenomenon. Perhaps leaving areas of the lung collapsed or at least ventilating them at low pressures might not damage the lung or the vasculature. Whether resting parts of the lung is better than trying to recruit the majority of the lung will require additional studies.
Historic Webb and Tierney Study
One of the original studies conducted by Webb and Tierney22 in the early 1970s showed that using inspiratory pressures of 45 cm of H2O without PEEP resulted in the rapid death of normal rats. Their study is frequently cited as evidence of the benefits of using protective ventilatory strategies. Interestingly, their discovery took nearly two decades to be recognized. In a 2003 editorial, Tierney29 wrote, “… we could hardly believe the results. It was as if we violated a thermodynamic law and got more out of it than we put into it. … Within minutes the rats were cyanotic and appeared moribund. … It took a decade or two for others to conclude that human lungs could be injured by such ventilation. … Our final paragraph 30 years ago suggested management … using protective ventilation and low tidal volumes.”
Role of PEEP in Lung Protection
In ALI, PEEP appears to provide some protection from tissue damage when high pressures are used. This is especially true if PEEP levels are greater than the opening pressure for recruitable alveoli. PEEP helps restore functional residual capacity (FRC) by recruiting previously collapsed alveoli. Adequate levels of PEEP prevent repeated collapse and reopening of alveoli and help maintain lung recruitment.14,27 However, if PEEP overinflates already patent alveoli, then increasing PEEP for a given VT may maximally stretch alveoli. This situation also may reduce cardiac output. Safely establishing an optimum PEEP level is not an exact science and can be challenging in critically ill patients. (Case Study 17-2; see Chapter 13 for additional information on setting PEEP.)46
To summarize, lung injury may occur as a result of either overdistention of the lungs or from repeated opening and closing of lung units throughout the respiratory cycle during mechanical ventilation.46 These two phenomena can result in shear stress and alveolar injury, edema formation, surfactant washout or alteration, microvascular injury, stretch injury, and biotrauma. Stretch injury and the associated biotrauma produces inflammatory mediators by lung tissue and leaking of these mediators into the circulation, where they have the potential of affecting distal organs and ultimately causing multiple-organ dysfunction syndrome.37 Research findings strongly support the concept of maintaining Pplateau at less than 30 cm H2O, setting low VT, and using enough PEEP to adequately maintain open alveoli in patients with ARDS to avoid lung injury from mechanical ventilation.47
Ventilator-Induced Respiratory Muscle Weakness
It is clear that delivering high airway pressures and volumes during mechanical ventilation can lead to damage to the lung parenchyma. Recent studies have shown that mechanical ventilation may also cause damage to the respiratory muscles.45 Specifically, imposing too little stress on the diaphragm during mechanical ventilation by lowering the demands on a patient’s respiratory muscles can induce respiratory muscle weakness.46
Laboratory studies using animal models have shown that prolonged controlled mechanical ventilation in which complete diaphragmatic inactivity occurs (i.e., no respiratory efforts are made and the mechanical ventilator performs all of the WOB) can lead to a significant decrease in the cross-sectional area of diaphragmatic fibers.47 More recent studies by Levine and colleagues involving human subjects support these findings.48 In their studies, Levine and colleagues obtained diaphragmatic muscle biopsies from mechanically ventilated patients who exhibited complete diaphragmatic inactivity for 18 to 69 hours. Histologic measurements of these muscle samples from the costal diaphragm revealed marked diaphragmatic atrophy. Biochemical analysis of the muscle samples suggested that the atrophy occurred as a result of increased oxidative stress and activation of protein-degradation pathways.48
The implications of these findings on clinical management of mechanically ventilated patients are unclear at this time. Additional clinical studies will be required to identify more completely the presence of ventilator-induced respiratory muscle weakness and its effect on weaning and ventilator discontinuation. Although respiratory muscle weakness can result from ventilator injury, it is important for clinicians to recognize that it can be associated with other medical conditions and interventions, such as sepsis and pharmacologic therapy (e.g., antibiotics, corticosteroids, sedatives, and neuromuscular blocking agents.46
Effects Of Mechanical Ventilation On Gas Distribution And Pulmonary Blood Flow
Ventilation to Nondependent Lung
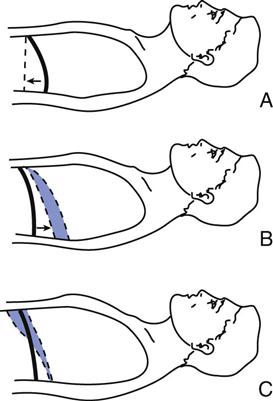
Early studies of the effects of positive-pressure breathing on the gas distribution in the normal lungs were conducted more than 30 years ago. Froese and Bryan49 evaluated the movement of the diaphragm in spontaneously breathing, anesthetized adult volunteers. During spontaneous ventilation in the supine position, the greatest displacement of the diaphragm occurs in the dependent region, near the back (Fig. 17-6, A).49 The dependent lung areas receive a higher portion of ventilation and perfusion (i.e., 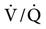 is best matched). When anesthesia is administered but spontaneous ventilation is still present, the diaphragm shifts its movement cephalad, toward the head. The effect of this shift is most pronounced in the dependent (dorsal) regions of the lung, the reverse of normal (Fig. 17-6, B). With anesthesia and the administration of paralytic agents, the contraction of the diaphragm is blocked. When PPV is provided, the diaphragm is most displaced in the nondependent regions of the lung (Fig. 17-6, C). The diaphragm becomes less compliant than the chest wall adjacent to the anterior part of the lungs in the supine patient. This alters the
is best matched). When anesthesia is administered but spontaneous ventilation is still present, the diaphragm shifts its movement cephalad, toward the head. The effect of this shift is most pronounced in the dependent (dorsal) regions of the lung, the reverse of normal (Fig. 17-6, B). With anesthesia and the administration of paralytic agents, the contraction of the diaphragm is blocked. When PPV is provided, the diaphragm is most displaced in the nondependent regions of the lung (Fig. 17-6, C). The diaphragm becomes less compliant than the chest wall adjacent to the anterior part of the lungs in the supine patient. This alters the 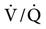 ratios by directing the greatest amount of gas flow to the nondependent lung regions, taking the path of least resistance. Unfortunately, this is also the area with the least blood flow.
ratios by directing the greatest amount of gas flow to the nondependent lung regions, taking the path of least resistance. Unfortunately, this is also the area with the least blood flow.
During PPV, alveolar collapse is suspected to most likely occur in the dependent areas with absence of spontaneous diaphragmatic movement. These are also the areas that receive the most blood flow, resulting in increased mismatching of ventilation and perfusion and increased dead space ventilation.49
Ventilation-to-Lung Periphery
Experimental studies have shown that during spontaneous ventilation, the distribution of gas favors the dependent lung areas and also appears to favor the periphery of the lung closest to the moving pleural surfaces. The peripheral areas receive more ventilation than the central areas.51,52 However, during a positive-pressure breath with passive inflation of the lung (paralysis), the central, upper airway, or peribronchial portions of the lung are preferentially filled with air.51 This may be another mechanism by which mismatching occurs during PPV. If spontaneous breathing can be preserved when possible, these changes in 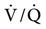 associated with mechanical ventilation may be reduced. Thus ventilator modes that preserve spontaneous breathing may be beneficial (e.g., pressure support ventilation [PSV]).
associated with mechanical ventilation may be reduced. Thus ventilator modes that preserve spontaneous breathing may be beneficial (e.g., pressure support ventilation [PSV]).
Increase in Dead Space
Positive-pressure ventilation increases the size of the conductive airways, which in turn increases the amount of dead space ventilation. Additionally, if normal alveoli are overexpanded during PPV and compression of pulmonary vessels results, alveolar dead space will also increase. On the other hand, if an increased VT is delivered and PPV improves ventilation distribution with respect to perfusion, then PPV will decrease the amount of dead space ventilation.
Redistribution of Pulmonary Blood Flow
Normal pulmonary blood flow favors the gravity-dependent areas and the central, or core, areas of the lungs. However, during PPV, particularly when PEEP is administered, cardiac output may decrease and pulmonary perfusion redistributes to the lung periphery rather than to the center area (i.e., as if the lung had been exposed to a centrifugal force).53 The clinical significance of this is unknown, but it may influence 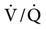 matching.
matching.
The increased volume during a positive-pressure breath and PEEP squeezes the blood out of nondependent zones, particularly in areas of normal lung. This further contributes to 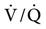 mismatching and physiological dead space by sending more blood into dependent areas, where ventilation is now lower, or into disease-affected areas of the lung, where lung volumes are not substantially increased. This can lead to increased shunting and decreased PaO2.54
mismatching and physiological dead space by sending more blood into dependent areas, where ventilation is now lower, or into disease-affected areas of the lung, where lung volumes are not substantially increased. This can lead to increased shunting and decreased PaO2.54
Conversely, improvement in 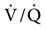 matching occurs when PEEP is applied to patients who have refractory hypoxemia resulting from a decreased FRC and increased shunting (i.e., ALI/ARDS). PEEP reduces intrapulmonary shunting resulting in an increase in PaO2. This increase in PaO2 implies improvement in
matching occurs when PEEP is applied to patients who have refractory hypoxemia resulting from a decreased FRC and increased shunting (i.e., ALI/ARDS). PEEP reduces intrapulmonary shunting resulting in an increase in PaO2. This increase in PaO2 implies improvement in 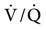 matching.2,6,55,56 A classic and predictable response of gas distribution and pulmonary perfusion during PPV apparently does not exist.
matching.2,6,55,56 A classic and predictable response of gas distribution and pulmonary perfusion during PPV apparently does not exist.
Effects of Positive Pressure on Pulmonary Vascular Resistance
As described previously, pulmonary perfusion may be compromised during PPV, especially when high levels of PEEP are also applied. Increased airway and alveolar pressures can lead to thinning and compression of pulmonary capillaries, decreased perfusion, and increased pulmonary vascular resistance (PVR) (Fig. 17-7). Fortunately, if expiration is prolonged and unimpeded (i.e., PEEP is not applied), the decreased pulmonary perfusion may be offset by normal flow back into the thorax during expiration with no net effect on PVR.
In most patients, severe hypoxia leads to increased PVR. This is caused by constriction of the pulmonary vessels and subsequent pulmonary hypertension. When mechanical ventilation improves oxygenation by opening up these capillary beds, pulmonary perfusion and PVR may actually improve.
At low lung volumes in which FRC is decreased, the addition of PEEP can potentially open collapsed alveoli, recruiting intraparenchymal (e.g., corner) vessels. This improves the 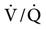 relations of the lungs. Thus, PPV has no clear effect with or without PEEP on PVR. Sometimes positive pressure reduces PVR, whereas at other times, it increases PVR.
relations of the lungs. Thus, PPV has no clear effect with or without PEEP on PVR. Sometimes positive pressure reduces PVR, whereas at other times, it increases PVR.
Respiratory And Metabolic Acid-Base Status In Mechanical Ventilation
The primary goal of mechanical ventilation is to maintain acceptable arterial blood gas (ABG) values in patients with compromised ventilatory function. Failure to achieve this goal occurs when the ventilator is not optimally adjusted or when adverse effects occur. Ventilatory problems associated with PPV can result in hypoventilation and hyperventilation. Patients may additionally demonstrate metabolic acid-base imbalances that can seriously affect their ventilatory management.
Hypoventilation
Acute hypoventilation can occur in patients receiving ventilatory support if adequate alveolar ventilation is not achieved. Hypoventilation will result in an elevated PaCO2 (i.e., hypercapnia) and an acidotic pH. Evaluation of clinical signs and symptoms, as well as ABG analysis, will lead to recognition of the problem.
Acidosis causes a right shift in the oxyhemoglobin dissociation curve and reduces the ability of hemoglobin to bind and carry oxygen at the lung. Additionally, in the absence of supplemental oxygen delivery, an increase in PaCO2 will lead to proportionate decreases in PaO2 and contribute to hypoxemia. If the patient already had hypoxemia, these factors may further reduce oxygenation. On the other hand, a right shift of the curve facilitates unloading of oxygen at the tissue level.
Rapidly rising PaCO2 levels and falling pH values can lead to serious problems, including coma. Elevated plasma hydrogen ion levels can contribute to high plasma potassium levels (hyperkalemia), which can affect cardiac function and can lead to cardiac dysrhythmias (Box 17-4). Hypercapnia also increases cerebral perfusion and can lead to increased intracranial pressure, which can be detrimental to patients with cerebral trauma, cerebral hemorrhage, or similar disorders.
On the other hand, in patients with ARDS, ventilation may be difficult to maintain without causing VILI. In these situations, permissive hypercapnia may be appropriate. In addition, hypercapnia may reduce the release of inflammatory mediators (see Chapter 13).42–44 Ultimately, the decision to allow respiratory acidosis to persist must be carefully evaluated on the basis of the patient’s condition.
The kidneys normally can compensate for respiratory acidosis within 18 to 36 hours. Obviously, it is desirable for problem to be corrected by increasing alveolar ventilation rather than waiting for renal compensation. Increasing ventilation can be accomplished by increasing the VT or mandatory rate.
When respiratory acidosis is present, patients receiving controlled mechanical ventilation may try to override the ventilator and take in a breath. They may not be able to trigger the machine or receive adequate flow and will appear to be fighting the ventilator. Increasing the sensitivity or flow will allow the patient to trigger the ventilator and receive an adequate breath. (See the discussion of ventilator asynchrony in this chapter.)
Hyperventilation
Hyperventilation results in a lower than normal PaCO2 and a rise in pH. Patient-induced hyperventilation is often associated with hypoxemia, pain and anxiety syndromes, circulatory failure, and airway inflammation. Ventilator-induced hyperventilation is generally caused by inappropriate ventilator settings. Alkalosis causes a left shift in the oxygen dissociation curve, which enhances the ability of hemoglobin to pick up oxygen in the lungs but makes it less available at the tissue level (i.e., the Haldane effect). Reduced hydrogen ion concentrations in the blood (i.e., arterial pH) are often accompanied by hypokalemia (low potassium levels), which can lead to cardiac arrhythmias (Box 17-5).