Through bound apolipoprotein A-I (apoA-I), high-density lipoprotein cholesterol (HDL-C) activates endothelial nitric oxide synthase, inducing vasodilation. Because patients with sickle cell disease (SCD) have low apoA-I and endothelial dysfunction, we conducted a randomized, double-blinded, placebo-controlled trial to test whether extended-release niacin (niacin-ER) increases apoA-I-containing HDL-C and improves vascular function in SCD. Twenty-seven patients with SCD with levels of HDL-C <39 mg/dl or apoA-I <99 mg/dl were randomized to 12 weeks of niacin-ER, increased in 500-mg increments to a maximum of 1,500 mg/day, or placebo. The primary outcome was the absolute change in HDL-C level after 12 weeks, with endothelial function assessed before and at the end of treatment. Niacin-ER-treated patients trended to greater increase in HDL-C level compared with placebo treatment at 12 weeks (5.1 ± 7.7 vs 0.9 ± 3.8 mg/dl, 1-tailed p = 0.07), associated with significantly greater improvements in the ratios of low-density lipoprotein to HDL-C levels (1.24 vs 1.95, p = 0.003) and apolipoprotein B to apoA-I levels (0.46 vs 0.58, p = 0.03) compared with placebo-treated patients. No improvements were detected in 3 independent vascular physiology assays of endothelial function. Thus, the relatively small changes in HDL-C levels achieved by the dose of niacin-ER used in our study are not associated with improved vascular function in patients with SCD with initially low levels of apoA-I or HDL-C.
Our laboratory has shown that patients with sickle cell disease (SCD) have significantly decreased high-density lipoprotein cholesterol (HDL-C) and apolipoprotein A-I (apoA-I) levels. We have also demonstrated that among patients with SCD, those with lower apoA-I levels have impaired vasodilatory responses to acetylcholine during forearm blood flow (FBF) strain gauge plethysmography and they tend to have elevated estimated pulmonary artery systolic pressures, an echocardiographic marker of pulmonary hypertension. In support of this finding, in patients with pulmonary arterial hypertension without SCD, plasma HDL-C levels are low and predict clinical worsening and death.
Extended-release niacin (niacin-ER) treatment promotes an increase in HDL-C level containing apoA-I. In particular, niacin-ER inhibits the degradation and hepatic clearance of apoA-I-containing HDL-C, cholesterol ester transport to low-density lipoprotein cholesterol, and also the actions of hepatic lipase. Interestingly, a precursor of niacin, nicotinic acid, was used in a case report in SCD with possible improvement in disease manifestations. We reasoned that niacin-ER administration would increase levels of HDL-C and apoA-I in SCD and improve vascular function and tested this hypothesis in a randomized, double-blinded, placebo-controlled trial. Our primary outcome was an absolute change in HDL-C level, and secondary outcomes included absolute change in apoA-I level and physiologic assessments of improved vascular function.
Methods
This was a prospective, single-center, randomized, double-blinded trial comparing niacin-ER with placebo treatment. The National Heart, Lung, and Blood Institute’s Institutional Review Board approved all protocols ( ClinicalTrials.gov identifier NCT00508989 ). All subjects provided written informed consent.
Subjects enrolled met all of the inclusion criteria: men or women 18 to 65 years of age, electrophoresis or high-performance liquid chromatographic documentation of homozygous hemoglobin S only phenotype, levels of HDL-C <39 mg/dl or apoA-I <99 mg/dl, level of hemoglobin >5.5 or <9.0 g/dl, and absolute reticulocyte count >95,000/μl. Subjects met none of the exclusion criteria: acute pain crisis requiring intravenous analgesics within 2 weeks before enrollment, women who were pregnant, lactating, or not using birth control at the time of enrollment, and hemoglobin SCD or hemoglobin A >20%. In addition, subjects were excluded if they used aspirin or nonsteroidal anti-inflammatory drugs within 1 week before vascular testing or used caffeine on the day of vascular testing. Pre-existing conditions that may independently affect endothelial function caused subjects to be excluded, including diabetes mellitus, cigarette smoking within 1 month before enrollment, renal failure, gout, and significant cardiovascular disease such as uncontrolled hypertension, peripheral artery disease, or severe hypotension. The use of medications including sildenafil, tadalafil, l -arginine, fibrates, inhaled nitric oxide, or any prostaglandins such as epoprostenol or treprostinil within 1 week before evaluation, or any statin within 4 weeks before enrollment caused subjects to be excluded.
Simple randomization was used to assign subjects to either 12 weeks of placebo or niacin-ER. The medication was incrementally dosed in 500-mg steps every 4 weeks as tolerated, to a maximum dose of 1,500 mg/day. Subjects were withdrawn if they developed: rhabdomyolysis (creatine kinase level of >5× the upper limit of normal), clinically significant myositis, red cell lysis, hepatocellular injury (alanine aminotransferase level of >3× the upper limit of normal or >123 mg/dl), elevated prothrombin time or partial thromboplastin time (to >1.5× control values), or intractable flushing unresponsive to ibuprofen therapy or dose reduction.
Our sample size calculation was based on a previous study of 11 subjects with coronary artery disease and initial HDL-C level of ≤36 mg/dl treated with niacin-ER (doses initiated at 375 mg and titrated up to 1,500 mg) for 12 weeks. The mean HDL-C level in this group increased from 30.1 to 40.5 mg/dl, with SD of 4 mg/dl both before and after treatment. Therefore, with α = 0.05 and a 98% power, the sample size needed to detect a mean difference of 10 mg/dl with SD = 4 mg/dl was 13 in each treatment group (JMP version 8.0, SAS Institute Inc., Cary, North Carolina). Our original protocol was designed to enroll 40 subjects with SCD who met eligibility criteria, 20 randomized to niacin-ER, and 20 to placebo. An interim analysis was planned when 12 subjects in each group completed the study.
To measure vascular and/or endothelial function, FBF, flow-mediated dilation (FMD) of the brachial artery, and peripheral arterial tonometry were performed at baseline and after 12 weeks of treatment. FBF was measured similarly to that by Gladwin et al. In brief, brachial artery and antecubital vein catheters were placed in the arm, with the intra-arterial catheter connected to a pressure transducer and an infusion pump that delivered 5% dextrose-in-water at 0.5 ml/min. After 20 minutes of rest, acetylcholine was infused at 7.5, 15, and 30 μg/min, sodium nitroprusside was infused at 0.8, 1.6, and 3.2 μg/min, and N G -monomethyl- l -arginine was infused at 4 μg/min. Each of the infusions was followed by a 30-minute washout with 5% dextrose-in-water infusion at 0.5 ml/min and repeat of a baseline measurement. After 3 minutes of each infusion dose (or 5 minutes for N G -monomethyl- l -arginine), FBF was measured. Infusions were done with acetylcholine to test endothelium-dependent vasodilation, sodium nitroprusside to test endothelium-independent vasodilation, and N G -monomethyl- l -arginine to measure basal nitric oxide production.
FMD and peripheral arterial tonometry (measured by endoPAT; Itamar Medical Ltd., Caesarea, Israel) involved occlusion-reperfusion to create a reactive hyperemia response. Using the method similar to that by Celermajer et al, FMD involved the use of an ultrasound probe to measure brachial artery diameter at baseline and during reperfusion after 5 minutes of occlusion by a sphygmomanometer inflated to suprasystolic pressures. FMD was calculated as the percent change in brachial artery diameter after release of the pressure in the sphygmomanometer or as (peak diameter − baseline diameter)/baseline diameter × 100%.
The endoPAT device involves a probe placed around a subject’s distal fingertip of the second phalange. As a sphygmomanometer is inflated around the subject’s upper arm, plethysmography is recorded. The endoPAT software calculates a reactive hyperemia index from the pulse-wave amplitude recorded at steady state and during reperfusion after 5 minutes of occlusion by a sphygmomanometer inflated to suprasystolic pressures. Reactive hyperemia index of ≤1.67 is considered an indicator of endothelial dysfunction.
Laboratory evaluations were performed in the Clinical Center Department of Laboratory Medicine at the National Institutes of Health by standard clinical laboratory assays, including standard complete blood counts with hemoglobin F levels, iron-binding studies, serum chemistry, lipid panels, amino terminal brain natriuretic peptide, homocysteine, and C-reactive protein levels. Soluble vascular cell adhesion molecule as well as nitrite (from plasma and whole blood) and nitrate levels were measured in our research laboratory. Blood samples were drawn every 4 weeks (or every 2 weeks if there was an elevated clinical concern for adverse effects and/or liver dysfunction). Pills were counted for compliance at each visit.
The prespecified primary outcome was the absolute change in HDL-C level after niacin-ER treatment, that is, post-treatment (week 12) minus pre-treatment (week 0) HDL-C levels. Changes in HDL-C levels were planned to be compared using a 1-sided Wilcoxon rank sum test because our primary hypothesis was that there would be a greater increase in HDL-C levels in the subjects taking niacin-ER. An interim analysis was planned after data were complete for 24 subjects, at a 2-sided 0.002 significance level. The following results are from the interim analysis, after which the study was stopped for futility. All p values are reported for the testing of a 2-tailed hypothesis, unless specified.
Results
A database of 305 patients was evaluated for eligibility: 27 adults with SCD were randomized to receive either placebo or niacin-ER daily for 12 weeks: 12 subjects received niacin-ER and 15 received placebo ( Figure 1 ). Three subjects were unable to complete the protocol, 2 in the niacin-ER treatment group and 1 in the placebo treatment group, because of reasons unrelated to study medication ( Figure 1 ). The main side effect reported was flushing, with 4 (27%) placebo-treated and 10 (83%) niacin-ER-treated subjects reporting at least 1 episode of flushing, despite using a placebo completely void of any niacin. Three subjects did not reach the target dose of 1,500 mg because of dose-dependent symptoms of flushing; 2 subjects in the niacin-ER group achieved a maximal dose of 1,000 mg and 1 only tolerated a dose no greater than 500 mg.

At baseline, subjects were similar on all variables except for a slight elevation in C-reactive protein level in the niacin-ER treatment group (placebo 0.2 [interquartile range 0.2 to 0.83] vs niacin-ER 0.89 [0.61 to 1.39] mg/dl, p = 0.048; Table 1 ).
| Variable | Placebo, n = 14 (mean ± SD or median [IQR]) | Niacin-ER, n = 10 (mean ± SD or median [IQR]) | p |
|---|---|---|---|
| Percent compliance (pill counts) | 92 (85–100) | 87 (82–92) | 0.34 |
| Tricuspid regurgitant velocity (m/s) | 2.2 ± 0.7 | 2.3 ± 0.2 | 0.95 |
| Alkaline phosphatase (U/L) | 65 (50–79) | 93 (69–110) | 0.11 |
| ALT (U/L) | 21 (16–27) | 31 (24–36) | 0.01 |
| AST (U/L) | 24 (18–33) | 37 (30–54) | 0.02 |
| C-reactive protein (mg/dl) | 0.5 (0.2–3.1) | 1.2 (0.5–4.6) | 0.36 |
| Lactate dehydrogenase (U/L) | 267 (209–302) | 323 (269–373) | 0.04 |
| sVCAM-1 (ng/ml) | 648 ± 242 | 1,024 ± 383 | 0.01 |
| Hemoglobin (g/dl) | 8.8 ± 1.2 | 9.2 ± 1.6 | 0.47 |
| Hemoglobin S (%) | 73.6 ± 14.6 | 84.4 ± 5.8 | 0.02 |
| Reticulocyte count (K/μl) | 200 ± 101 | 199 ± 84 | 0.98 |
| White blood cell count (K/μl) | 7.9 ± 2.8 | 7.9 ± 1.7 | 0.99 |
| Systolic blood pressure (mm Hg) | 107 (103–117) | 117 (112–125) | 0.03 |
| Diastolic blood pressure (mm Hg) | 63 (56–73) | 68 (63–75) | 0.24 |
| HDL-C (mg/dl) | 31 ± 3.6 | 37 ± 12 | 0.11 |
| LDL-C (mg/dl) | 57 (52–66) | 51 (33–62) | 0.21 |
| LDL-C/HDL-C ratio | 1.95 (1.70–2.10) | 1.24 (1.04–1.51) | 0.003 |
| apoA-I (mg/dl) | 96 (80–108) | 104 (87–110) | 0.62 |
| apoB (mg/dl) | 54 (48–65) | 48 (44–50) | 0.07 |
| apoB/apoA-I ratio | 0.58 (0.47–0.68) | 0.46 (0.4–0.49) | 0.03 |
| Cholesterol (mg/dl) | 109 (98–119) | 104 (77–129) | 0.75 |
| Triglycerides (mg/dl) | 89 ± 48 | 78 ± 36 | 0.55 |
There were few differences between treatment groups in complete blood count or other lipid panel levels after 12-week treatment. The difference in hemoglobin S level is likely caused by differences in clinically indicated blood transfusion. Throughout 12 weeks, 4 placebo-treated subjects required a total of 6 transfusions, whereas in the niacin-ER-treated group, no subjects required transfusion ( Figure 1 ). This is an intriguing, although preliminary, finding. Compliance with study medication did not significantly differ between treatment groups.
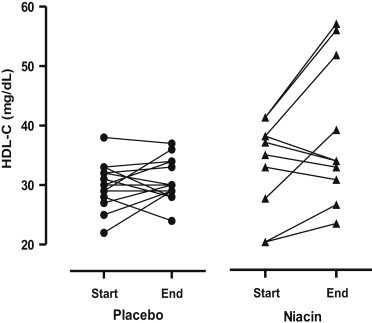
After 12 weeks, all niacin-ER-treated subjects had an increase in HDL-C levels from baseline, although the increase was smaller than expected and nonsignificant (mean change in HDL-C level for placebo 0.9 ± 3.8 vs niacin-ER 5.1 ± 7.7 mg/dl, 1-tailed p = 0.07; percent change in HDL-C level for placebo 3.9 ± 14.0% vs niacin-ER 16.2 ± 21.8%), resulting in a negative primary outcome ( Figure 2 ). Four of the niacin-ER-treated subjects had substantial increases in HDL-C levels of 11 to 15 mg/dl (35% to 41% increase). The remaining 6 niacin-ER-treated subjects who completed the study had an increase in HDL-C levels of <10 mg/dl.