Reports differ regarding the effect of concomitant coronary artery bypass grafting (CABG) in patients who undergo aortic valve replacement (AVR) for aortic stenosis (AS), and no reports have described the effect of aortic valve structure in patients who undergo AVR for AS. A total of 871 patients aged 24 to 94 years (mean 70) whose AVR for AS was their first cardiac operation, with or without first concomitant CABG, were included. Patients who underwent mitral valve procedures were excluded. In comparison with the 443 patients (51%) who did not undergo CABG, the 428 (49%) who underwent concomitant CABG were significantly older, were more often male, had lower transvalvular peak systolic pressure gradients and larger valve areas, had lower frequencies of congenitally malformed aortic valves, had lighter valves by weight, had higher frequencies of systemic hypertension, and had longer stays in the hospital after AVR. Early and late (to 10 years) mortality were similar by propensity-adjusted analysis in patients who did and did not undergo concomitant CABG. Congenitally unicuspid or bicuspid valves occurred in approximately 90% of those aged 21 to 50, in nearly 70% in those aged 51 to 70 years, and in just over 30% in those aged 71 to 95 years. Unadjusted and adjusted survival was significantly higher in patients with unicuspid or bicuspid valves compared to those with tricuspid valves. In conclusion, although concomitant CABG had no effect on the adjusted probability of survival, the type of aortic valve (unicuspid or bicuspid vs tricuspid) significantly affected the unadjusted and adjusted probability of survival.
Adults with isolated (no mitral valve disease) aortic stenosis (AS) (with or without aortic regurgitation) frequently have associated narrowing of ≥1 major (right, left main, left anterior descending, or left circumflex) epicardial coronary arteries. In reported patients who undergo aortic valve replacement (AVR) for AS, the effect of concomitant coronary artery bypass grafting (CABG) on early and late mortality has been variable. The aim of this study was to compare among patients with isolated AS the outcomes of AVR in patients with versus without CABG and also the effect of aortic valve structure (unicuspid or bicuspid vs tricuspid) on the probability of long-term survival after AVR for AS.
Methods
The surgical pathology files of the cardiovascular laboratory, a part of the pathology department at Baylor University Medical Center (BUMC) in Dallas, were searched for patients having operatively excised aortic valves without simultaneous replacement of the mitral valve or evidence of mitral stenosis. From January 2002 through June 2010 (102 months [8.5 years]), a total of 1,040 operatively excised stenotic aortic valves were submitted to the cardiovascular laboratory at BUMC. Each was described, and the surgical report was prepared by W.C.R. and photographed by J.M.K. The valve structure in each was determined by W.C.R from examination of the operatively excised aortic valve. This study was approved by the Institutional Review Board at BUMC on May 5, 2010, and the requirement for individual patient consent was waived.
Included in the present study were 871 adults whose AVR for AS was their first cardiac operation, with or without first simultaneous CABG. Patients who underwent isolated AVR for AS and later in a second operation underwent CABG or vice versa were excluded.
Clinical records, including admission note, discharge summary, echocardiographic and cardiac catheterization reports, and operative records, were sought from the patients’ medical charts and/or BUMC Apollo cardiovascular database (Apollo Advance c/s version 4.2.13; LUMEDX Corporation, Oakland, California). (The Apollo system became operational at BUMC in January 2002.)
Echocardiographic and hemodynamic data were obtained from the cardiovascular computer database. Whether simultaneous CABG was performed at the time of AVR was also obtained from either the computer database or from the medical records. Information regarding the death of any patient was obtained from 3 sources: (1) the medical records in the deaths during the hospitalization at the time of AVR, (2) the Society of Thoracic Surgeons National Database, and (3) the Social Security Death Index for posthospitalization deaths. Echocardiographic data preoperatively were available for 426 patients (49%). Cardiac catheterization data preoperatively were available for 561 patients (64%).
Means, standard deviations, and percentages were calculated to describe the study cohort (n = 871). Differences in demographic, clinical, and morphologic details were tested using Wilcoxon’s tests (for continuous variables) or chi-square tests (for categorical variables). Bonferroni’s correction was used to account for multiplicity. Unadjusted p values, odds ratios, hazard ratios, 95% confidence intervals (CIs), and log-rank statistics were estimated to describe 30-day and long-term mortality. Kaplan-Meier plots were used to describe unadjusted survival in patients who underwent concomitant CABG compared to those who did not and in patients with unicuspid or bicuspid valves compared to those with tricuspid valves.
A propensity score approach was used to assess the adjusted association between undergoing concomitant CABG (vs not) and 30-day and long-term mortality. To estimate the propensity score, we used a logistic regression model with receiving concomitant CABG (vs not) as the dependent variable and age, gender, race, body mass index, aortic valve structure, valve weight, systemic hypertension, ascending aorta replaced, and type of implanted valve as independent variables. Restricted cubic splines were used for all continuous variables. Multiple imputation via predictive mean matching was used to account for missing data regarding the independent variables in this model. Estimates from the aforementioned propensity model were then used to adjust the effect of concomitant CABG on 30-day mortality in a logistic regression model and on long-term mortality in a Cox proportional-hazards model. Possible effect modification by gender and age was investigated for the 2 models and ruled out. The Grambsch and Therneau test statistic was used to test for proportionality of hazards in the Cox model.
The same approach, controlling for the same confounding factors except that valve structure was replaced by concomitant CABG, was used to assess the adjusted association between valve structure (unicuspid or bicuspid vs tricuspid valve structure) and 30-day and long-term mortality.
Statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina) and R software version 2.12.1 (R Project for Statistical Computing, Vienna, Austria).
Results
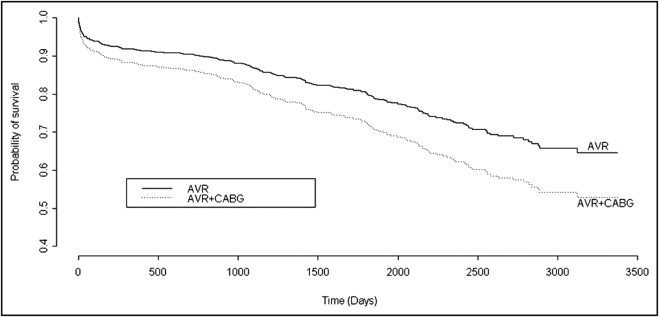
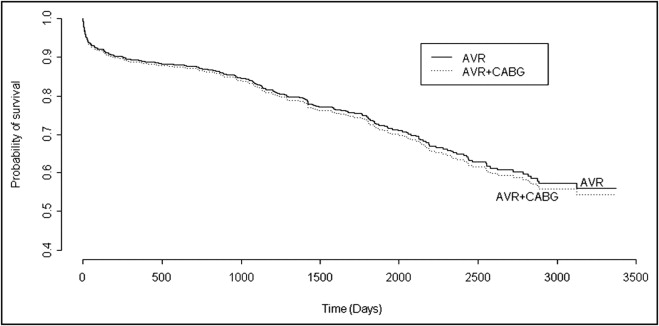
Demographic, clinical, and morphologic findings in the 871 patients included in the present study and their comparison between those with versus without concomitant CABG are listed in Table 1 . Of the 871 patients, 428 (49%) underwent concomitant CABG, and 443 (51%) did not. In comparison to the group that did not undergo CABG, those who underwent concomitant CABG were significantly older, were more often male, had lower transvalvular peak systolic pressure gradients and larger valve areas, had higher frequencies of tricuspid aortic valves and lower frequencies of congenitally malformed aortic valves ( Figure 1 ) , had lighter aortic valves by weight, had higher frequencies of systemic hypertension, and had longer stays in the hospital after AVR ( Table 1 ). Unadjusted early mortality (30 days) was not significantly (odds ratio 1.59, 95% CI 0.89 to 2.85) different between those who underwent (29 deaths [7%]) and those who did not undergo (18 deaths [4%]) simultaneous CABG. The length of stay for patients surviving >30 days and long-term survival (up to 10-year follow-up) were significantly higher in those who underwent concomitant CABG (median length of stay 8 days vs 6 days for patients who did not undergo concomitant CABG, p = 0.001; unadjusted hazard ratio for survival 1.46, 95% CI 1.12 to 1.91, log-rank p = 0.005). The unadjusted survival curves are depicted in Figure 2 . However, after adjustment for possible confounders (age, gender, race, body mass index, aortic valve structure, valve weight, systemic hypertension, ascending aorta replaced, and type of implanted valve), neither early mortality (30 days; adjusted odds ratio 1.69, 95% CI 0.87 to 3.29) nor long-term mortality (10-year follow-up; adjusted hazard ratio 1.01, 95% CI 0.74 to 1.34) was different between those who did and did not undergo simultaneous CABG. The adjusted long-term survival curves are depicted in Figure 3 .
| Variable | Coronary Artery Bypass Grafting | ||
|---|---|---|---|
| Yes (n = 428 [49%]) | No (n = 443 [51%]) | p Value | |
| Age group (years) | <0.001 | ||
| 21–50 | 12 (3%) | 54 (12%) | |
| 51–70 | 139 (32%) | 201 (45%) | |
| 71–95 | 277 (65%) | 188 (42%) | |
| Gender | <0.001 | ||
| Male | 289 (68%) | 233 (53%) | |
| Female | 139 (32%) | 210 (47%) | |
| Race | 0.953 | ||
| White | 354 (83%) | 367 (83%) | |
| Black | 24 (6%) | 22 (5%) | |
| Hispanic | 16 (4%) | 19 (4%) | |
| Other or unknown | 34 (8%) | 35 (8%) | |
| Number of major coronary arteries narrowed ≥50% | (n = 205) | (n = 272) | <0.001 |
| 0 | 32 (16%) ⁎ | 246 (90%) | |
| 1 | 60 (29%) | 20 (7%) | |
| 2 | 68 (33%) | 5 (2%) | |
| 3 | 34 (17%) | 1 (<1%) | |
| 4 | 11 (5%) | 0 | |
| LV-aorta peak systolic gradient (mm Hg) (n = 475), range (mean) [median] | 10–119 (46) [41] | 11–154 (54) [52] | <0.001 |
| Aortic valve area (cm 2 ) (n = 474), mean ± SD | 0.85 ± 0.31 | 0.76 ± 0.25 | 0.008 |
| Aortic valve structure | <0.001 | ||
| Unicuspid | 11 (3%) | 49 (11%) | |
| Bicuspid | 142 (33%) | 243 (55%) | |
| Tricuspid | 275 (64%) | 151 (34%) | |
| Aortic valve weight (g) | <0.001 | ||
| Unicuspid, range (mean) [median] | 1.20–4.78 (2.89) [2.71] | 1.19–7.15 (3.47) [3.28] | |
| Bicuspid, range (mean) [median] | 0.67–18.38 (3.11) [2.62] | 0.81–9.68 (3.11) [2.91] | |
| Tricuspid, range (mean) [median] | 0.43–6.40 (2.00) [1.84] | 0.55–5.63 (2.11) [1.98] | |
| Systemic hypertension by history | 324 (76%) | 292 (66%) | 0.015 |
| Body mass index (kg/m 2 ) | 0.872 | ||
| ≤25 | 148 (35%) | 140 (32%) | |
| >25–30 | 156 (37%) | 136 (31%) | |
| >30–40 | 104 (24%) | 141 (32%) | |
| >40 | 18 (4%) | 22 (5%) | |
| Range (mean ± SD) | 15–69 (28 ± 6) | 15–61 (29 ± 6) | |
| Days in hospital postoperatively in patients living >30 days, range (mean) [median] | 3–53 (10) [8] | 3–71 (8) [6] | 0.001 |
| Died ≤30 days postoperatively | 29/427 (7%) | 18/437 (4%) | 0.179 |
| Ascending aorta replaced | 11 (3%) | 26 (6%) | 0.159 |
| Implanted valve type | <0.001 | ||
| Mechanical | 64 (16%) | 140 (32%) | |
| Bioprosthetic | 355 (84%) | 289 (67%) | |
| Ross procedure | 1 (<1%) | 6 (1%) | |
⁎ These patients had significant narrowing of a branch of ≥1 major coronary artery.
The valve structure in the 871 patients is listed in Table 2 . The 426 patients (49%) with tricuspid aortic valves, compared to the 445 patients (51%) with either congenitally unicuspid or bicuspid valves, were significantly older (p <0.001), had much higher frequencies of concomitant CABG (p <0.001), had higher likelihoods of having ≥1 major epicardial coronary artery being narrowed >50% in diameter on angiogram (p <0.001), had lower transvalvular peak systolic pressure gradients (p <0.001), had lighter aortic valves by weight (p <0.001), had higher frequencies of systemic hypertension (p <0.001), had more days in the hospital after AVR (p <0.001), and had higher late (>30 days) mortality rates after AVR. Unadjusted early mortality (30 days) was not significantly (odds ratio 1.16, 95% CI 0.56 to 2.42) different between patients with unicuspid or bicuspid versus tricuspid valves. Both unadjusted and adjusted survival, however, were significantly higher in patients with unicuspid or bicuspid valves compared to patients with tricuspid aortic valves (adjusted hazard ratio 0.70, 95% CI 0.50 to 0.97; Figures 4 and 5 ) .
| Variable | Aortic Valve Structure | p Value | ||
|---|---|---|---|---|
| Unicuspid (n = 60 [7%]) | Bicuspid (n = 385 [44%]) | Tricuspid (n = 426 [49%]) | ||
| Age group (years) | <0.001 | |||
| 21–50 | 31 (52%) | 29 (8%) | 6 (1%) | |
| 51–70 | 24 (40%) | 206 (54%) | 110 (26%) | |
| 71–95 | 5 (8%) | 150 (39%) | 310 (73%) | |
| Gender | 0.169 | |||
| Male | 36 (60%) | 252 (65%) | 234 (55%) | |
| Female | 24 (40%) | 133 (35%) | 192 (45%) | |
| Race | 0.274 | |||
| White | 47 (78%) | 324 (84%) | 350 (82%) | |
| Black | 2 (3%) | 12 (3%) | 32 (8%) | |
| Hispanic | 4 (7%) | 18 (5%) | 13 (3%) | |
| Other | 7 (12%) | 31 (8%) | 31 (7%) | |
| Coronary artery bypass grafting | 11 (18%) | 142 (37%) | 275 (65%) | <0.001 |
| Number of major coronary arteries narrowed ≥50% | (n = 29) | (n = 212) | (n = 236) | <0.001 |
| 0 | 23 (79%) | 143 (67%) | 102 (43%) | |
| 1 | 3 (10%) | 34 (16%) | 52 (22%) | |
| 2 | 2 (7%) | 23 (11%) | 49 (21%) | |
| 3 | 1 (3%) | 8 (4%) | 26 (11%) | |
| 4 | 0 | 4 (2%) | 7 (3%) | |
| LV-aorta peak systolic gradient (mm Hg) (n = 475), range (mean) [median] | 20–154 (64) [63] | 11–130 (55) [51] | 11–119 (45) [41] | <0.001 |
| Aortic valve area (cm 2 ) (n = 474), mean ± SD | 0.80 ± 0.34 | 0.78 ± 0.25 | 0.83 ± 0.30 | 0.139 |
| Aortic valve weight (g), range (mean) [median] | 1.19–7.15 (3.37) [3.17] | 0.67–18.38 (3.11) [2.79] | 0.43–6.40 (2.04) [1.92] | <0.001 |
| Systemic hypertension by history | 27 (45%) | 260 (68%) | 329 (77%) | <0.001 |
| Body mass index (kg/m 2 ) | 0.863 | |||
| ≤25 | 24 (41%) | 119 (31%) | 145 (34%) | |
| >25–30 | 21 (36%) | 130 (34%) | 141 (33%) | |
| >30–40 | 13 (22%) | 111 (29%) | 121 (29%) | |
| >40 | 1 (2%) | 22 (6%) | 17 (4%) | |
| Range (mean ± SD) | 17–44 (27 ± 5) | 16–66 (29 ± 7) | 15–69 (29 ± 6) | |
| Days in hospital postoperatively in patients living >30 days, range (mean) [median] | 3–32 (7) [6] | 3–43 (8) [6] | 3–71 (10) [8] | <0.001 |
| Died ≤30 days postoperatively | 0/59 | 20/380 (5%) | 27/425 (6%) | 0.222 |
| Ascending aorta replaced | 9 (15%) | 25 (6%) | 3 (1%) | <0.001 |
| Implanted valve type | <0.001 | |||
| Mechanical | 41 (68%) | 118 (31%) | 53 (12%) | |
| Bioprosthetic | 17 (28%) | 264 (69%) | 371 (87%) | |
| Ross procedure | 2 (3%) | 3 (<1%) | 2 (<1%) | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


