Table 24.1 Appropriateness Criteria for the Application of Echocardiography in systemic Disease and Clinical decision Making | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Table 24.2 Systemic diseases and Clinical Presentations in Which Echocardiography Plays a Valuable Role | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
reported in preclinical infiltrative and hypertrophic cardiomyopathies and are likely present in a broad range of othe disease states as well. As such, their utilization clearly needs to be put in context of the clinical situation.
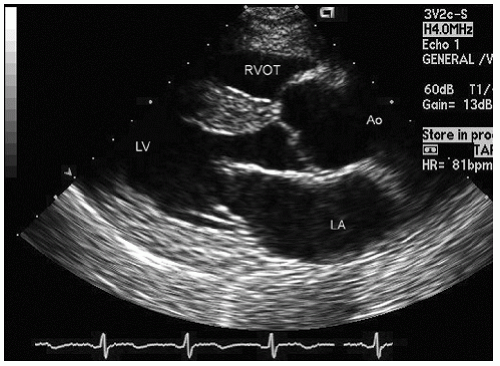
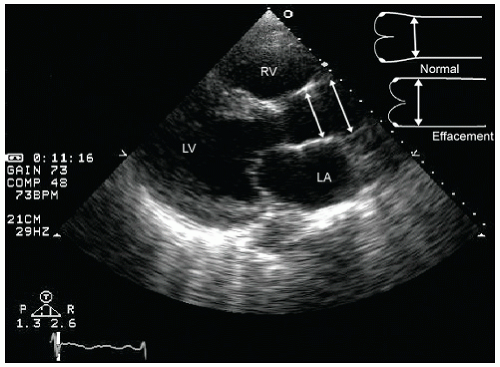
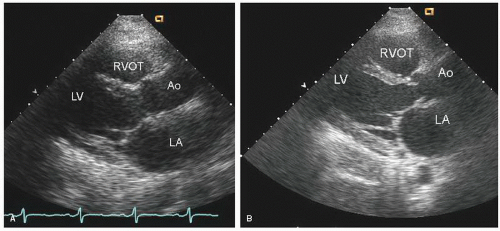
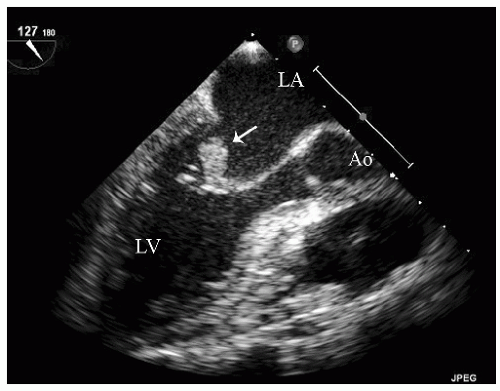
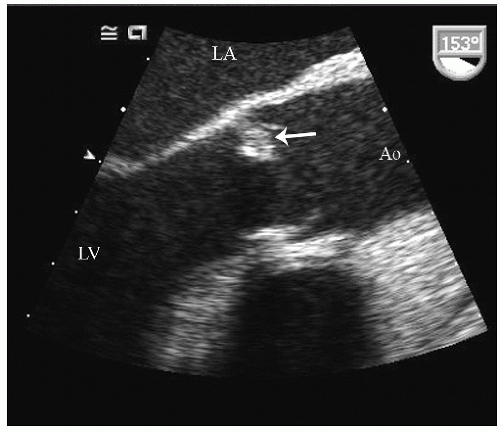
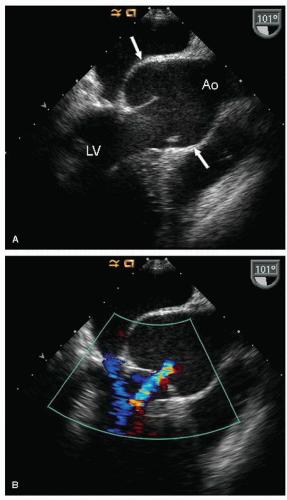
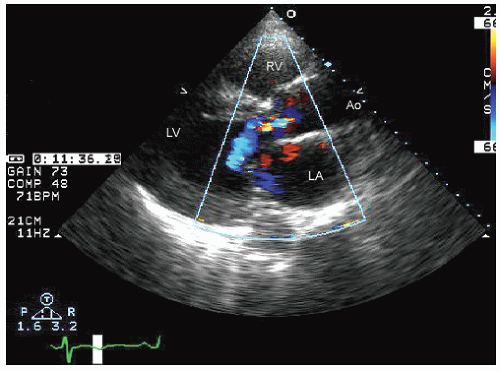 FIGURE 24.3. Parasternal long-axis echocardiogram with color Doppler flow imaging recorded from the same patient presented in Figure 24.2. Note the effacement of the sinotubular junction, which results in malcoaptation of the aortic cusps and a central aortic regurgitation jet. |
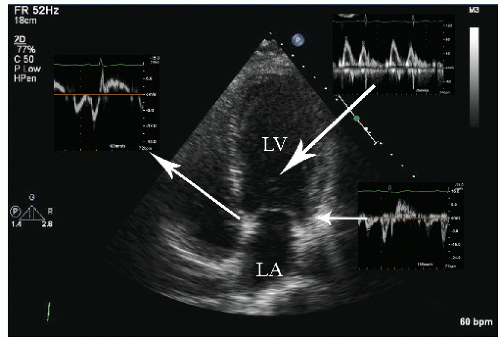
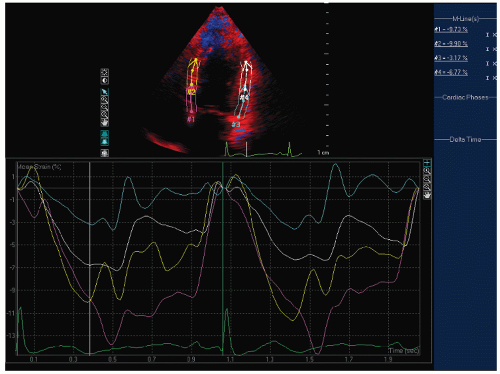 FIGURE 24.6. Doppler tissue based strain imaging recorded from the same patient depicted in Figure 24.5. The strain images reveal reduced mean strain predominantly in the lateral wall with a lesser reduction in the two septal segments. |
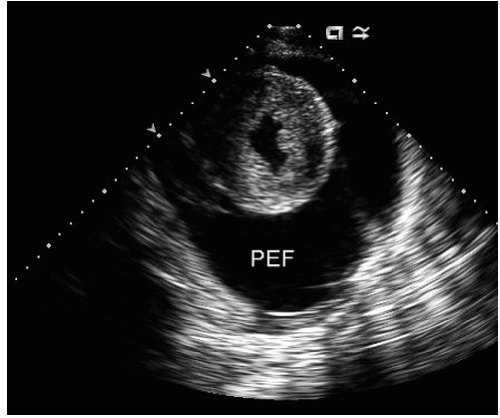
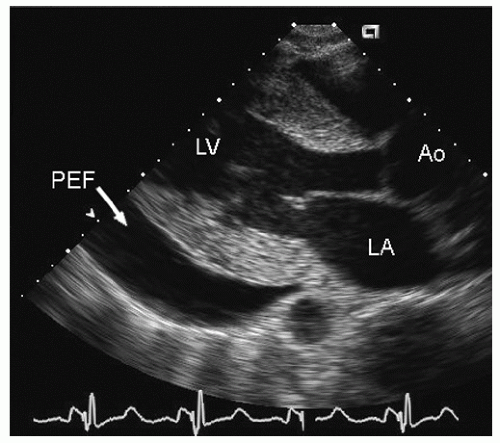
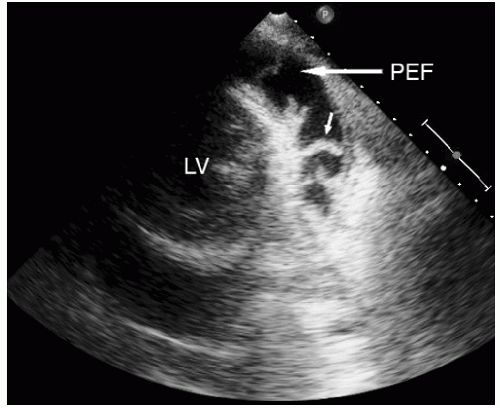
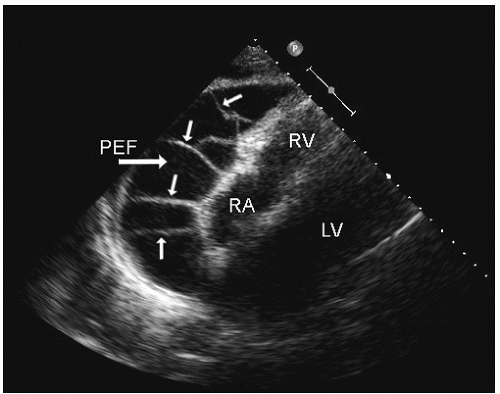
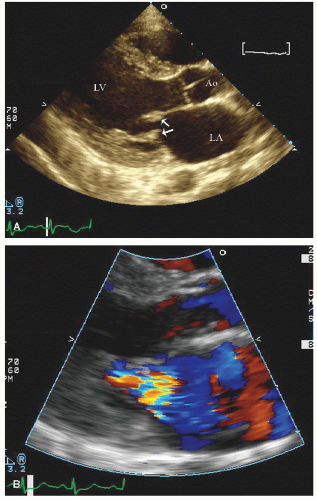
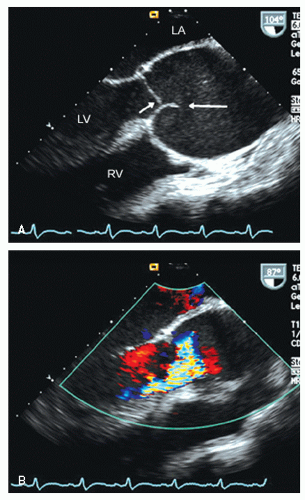
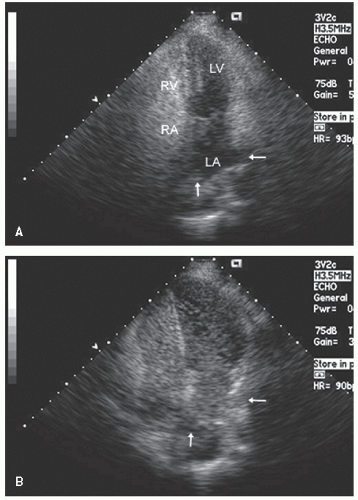
include left ventricular hypertrophy due to hypertension and an abnormal texture of the hypertrophied myocardium that mimics that seen in cardiac amyloid (Fig. 24.10). Other abnormalities seen in chronic renal insufficiency include pericardial effusion, which may range from small chronic effusions to presentation with cardiac tamponade. Uremia results in inflammatory and occasionally hemorrhagic pericarditis in which there is often evidence of “stranding” on the visceral pericardium (Figs. 24.11 and 24.12).
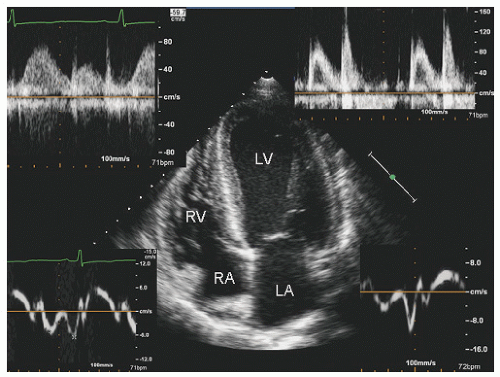
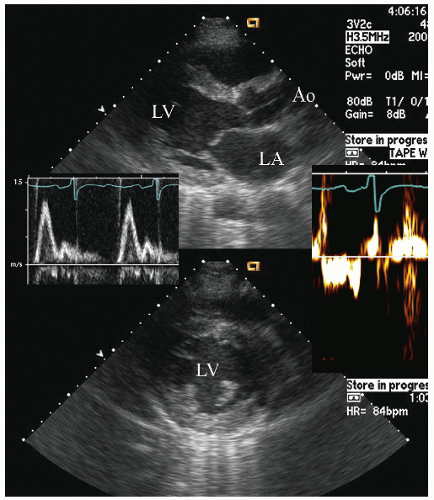
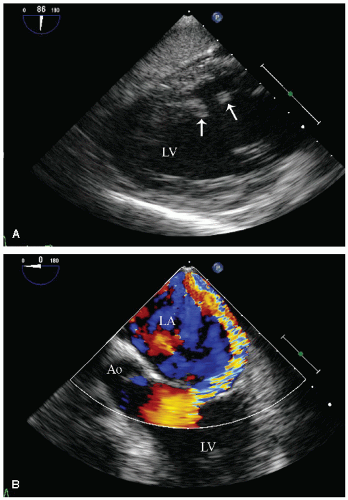
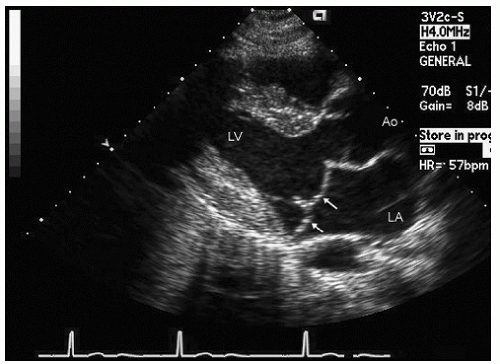
reported in which systolic function recovers after institution of more aggressive dialysis or renal transplantation. Figure 24.13 was recorded in a 34-year-old patient with end-stage renal disease related to glomerulonephritis. Note the significant systolic dysfunction in the real-time images and evidence of marked diastolic dysfunction. Figure 24.14 was recorded 6 months after renal transplantation and demonstrates marked reversal of both the systolic and diastolic dysfunction.
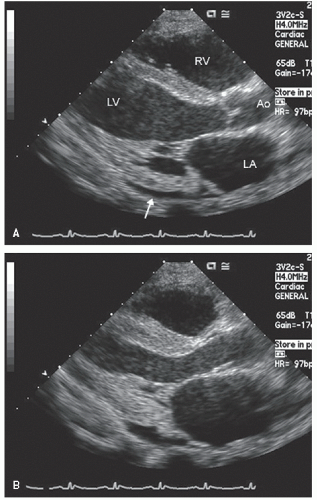
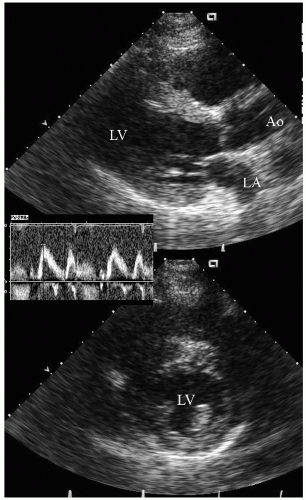 FIGURE 24.14. Parasternal long- and short-axis echocardiogram recorded 6 months after renal transplantation in the same patient depicted in Figure 24.13. In the real-time images, note the almost complete recovery of systolic function. Also note the normalization of mitral inflow. |
are no characteristic features of the pericarditis or pericardial infusion seen in SLE. On rare occasion, SLE has been associated with pulmonary hypertension, although this association is far more common with scleroderma.
primary pulmonary hypertension with an increase in pulmonary vascular resistance at the arteriolar level (Fig. 24.19). Concurrent pericardial effusion may be more common in scleroderma than in pulmonary hypertension of other etiologies and is not necessarily an indicator of end-stage disease. The manifestations of pulmonary hypertension as a distinct entity are discussed further in this chapter, and the echocardiographic features of right ventricular pressure overload have been discussed in Chapters 8 and 13.
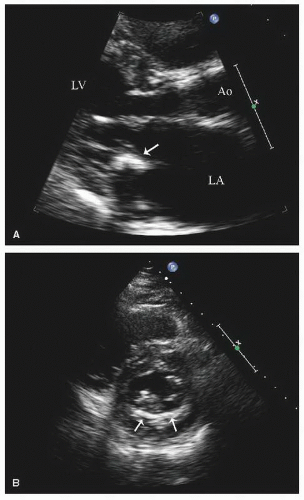
as a cause of aortic insufficiency. Dilation of the actual aortic annulus is uncommon, and in most patients, aortic insufficiency is the result of effacement of the sinotubular junction and not an abnormality of the annulus.
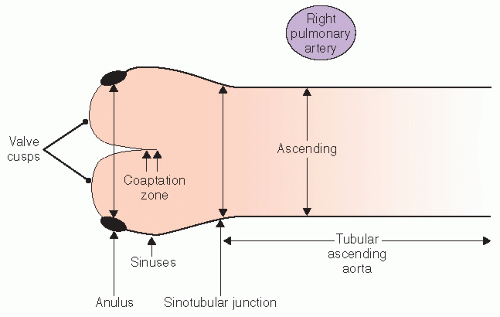 FIGURE 24.21. Schematic representation of normal aortic anatomy and the different components of the proximal aorta as well as recommended sites for making measurements. |
a patient should undergo an evaluation of the entire extent of at least the thoracic aorta, which can be performed with transesophageal echocardiography, computed tomography, or magnetic resonance imaging. If there is no evidence of distal aortic dilation, follow-up usually can be performed with transthoracic echocardiography because the proximal ascending aorta is the single most likely site to be involved in subsequent dilation. It should be emphasized that follow-up should include serial measurements as noted previously for comparison. A maximum aortic dimension of 55 mm is considered an indication for elective surgical intervention. However, a threshold of 50 mm has been recommended in the presence of a bicuspid aortic valve or in the Marfan syndrome and is also used as a general indication in high-volume centers. In addition, an interval increase in size of 5 mm over a period of 12 months or less is considered an indication for prophylactic aortic replacement. The need to index aortic size to body size is not firmly established; however, the implications of dilation less than 55 mm in a smallstatured individual are obvious. Aortic dilation associated with clinically relevant aortic insufficiency has been considered an indication for surgery as well. After surgical repair, continued surveillance is crucial because this is a systemic process involving all portions of the aorta. However, after replacement of the ascending aorta in a patient with Marfan syndrome, follow-up may require transesophageal echocardiography, computed tomography, or magnetic resonance imaging because additional disease will typically not be in the field of view of transthoracic echocardiography.
pressures with hepatic congestion result in an obstructive biochemical pattern.
Table 24.3 Heart and Liver Disease | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
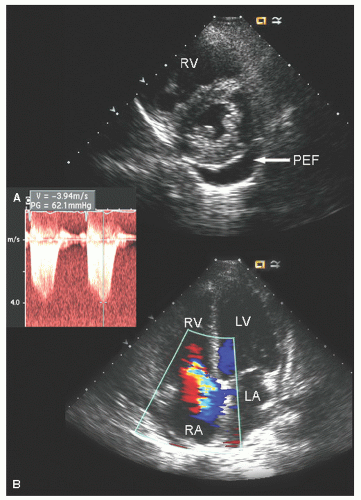
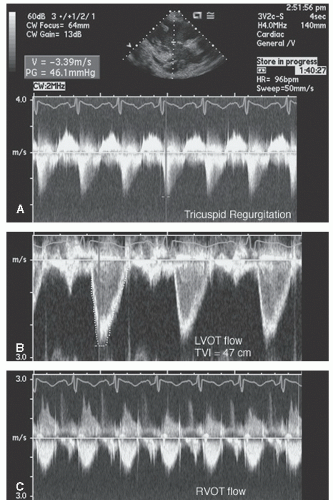 FIGURE 24.25. Spectral Doppler imaging recorded in the patient presented in Figure 24.24. Note the peak tricuspid regurgitation velocity of 3.4 m/sec and the greater than usual time velocity integral (TVI)of both left ventricular outflow tract and right ventricular outflow tract. |
pathologic right-to-left shunt due to pulmonary AVMs. If the magnitude of shunting is significant, percutaneous closure of the pulmonary AVM may be beneficial. Identification of such a shunt also assists in clinical management because it may provide an explanation for otherwise unexplained arterial desaturation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree