While transthoracic echocardiography provides only a limited view of the proximal ascending aorta and a small portion of the descending aorta and arch, transesophageal echocardiography provides a high-resolution view of the aorta from the aortic valve to approximately the diaphragm. Both normal and pathologic states can be identified with an accuracy equivalent to that of the competing techniques of computed tomography (CT) and magnetic resonance imaging (MRI). The speed with which this can be accomplished, often on a portable basis in an intensive care unit, confers obvious advantages with respect to the emergency evaluation of aortic dissection, suspected aortic trauma, or in the critically ill. Transthoracic echocardiography plays a valuable role for screening and serial follow-up of diseases such as Marfan syndrome, which may predominantly affect the proximal ascending aorta. Multiple diseases affect different portions of the aorta. These are outlined in
Table 21.1 and include dilation (annuloaortic ectasia) and aneurysm formation, atherosclerosis, acute and chronic dissection, coarctation, and various forms of arteritis. ACC/AHA guidelines have defined the appropriate use of echocardiography for the evaluation of known or suspected disease of the aorta in different clinical situations (
Table 21.2).
Normal Aortic Anatomy
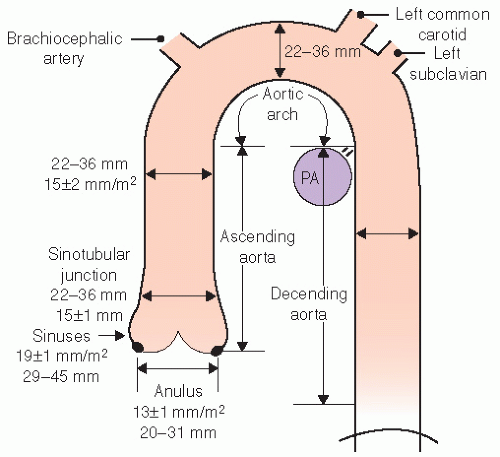
The normal aorta consists of six segments. These are schematized in
Figures 21.1 and
21.2 and consist of the annulus, sinuses of Valsalva, sinotubular junction, ascending tubular aorta, the arch, and the descending thoracic aorta. The proximal portion, from the annulus to the proximal ascending aorta, is commonly referred to as the “aortic root.” The aortic annulus is defined as the junction of the proximal ascending aorta with the left ventricular outflow tract. It is part of the fibrous skeleton of the heart and is contiguous with the anterior mitral valve leaflet and perimembranous septum. Because the annulus is a fibrous structure, it is relatively resistant to dilation and represents a relatively stable dimension to which the remaining aortic sizes can be indexed. Typically, the aortic annulus measures 13 ± 1.0 mm/m
2. The normal aorta dilates at the level of the sinuses by approximately 6 mm/m
2 and then tapers to within 2 to 3 mm of annular size at the sinotubular junction (
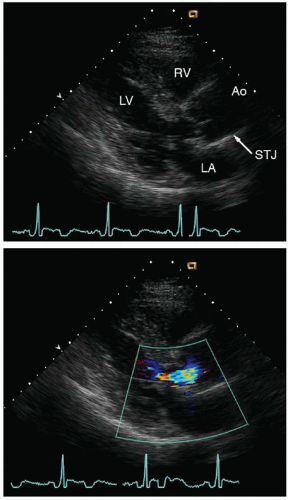
Fig. 21.1). Aortic size is related to height and body surface area. Normally there are three sinuses of Valsalva of equal size. The right and left sinuses contain the ostia of the right and left coronary arteries. The takeoff of the left coronary artery can be visualized by both transthoracic and transesophageal echocardiography in the left sinus where its position is relatively closer to the annulus than is the takeoff of the right coronary artery, which tends to be more superior and closer to the sinotubular junction.
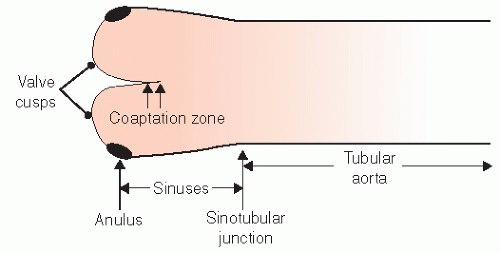
The geometry of the sinotubular junction is a crucial feature of normal aortic valve coaptation. Insertion of aortic valve cusps is continuous from the level of the annulus up through the sinuses to the sinotubular junction. Dilation of the sinotubular junction can result in splaying of the coaptation line of the aortic
cusps, resulting in secondary aortic insufficiency. The ascending aorta terminates at the right innominate artery (brachiocephalic artery), where the aortic arch begins and continues to the left subclavian artery and ligamentum arteriosum. The three major branch vessels of the arch, the right innominate artery, and the left carotid and subclavian arteries can be visualized in most patients from a suprasternal view as well as from the transesophageal approach. The dimension of the ascending aorta, arch, and descending thoracic aorta are all similar with slight tapering in the descending thoracic aorta.
Echocardiographic Evaluation
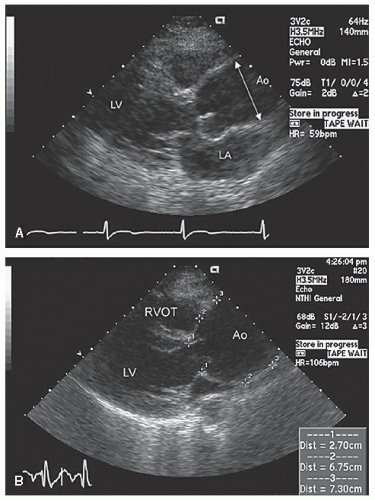
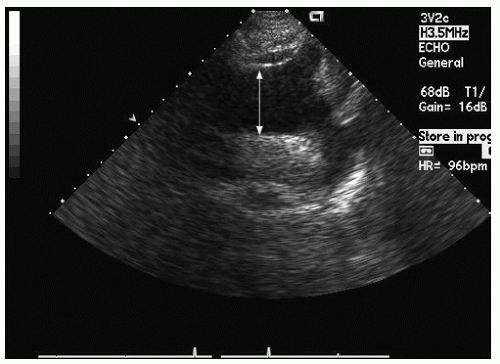
Only the proximal 4 to 8 cm of the ascending aorta, the arch, and a short segment of the descending thoracic aorta reliably can be evaluated with transthoracic echocardiography. Typically, the proximal aorta can be seen in its long and short axis from the parasternal view.
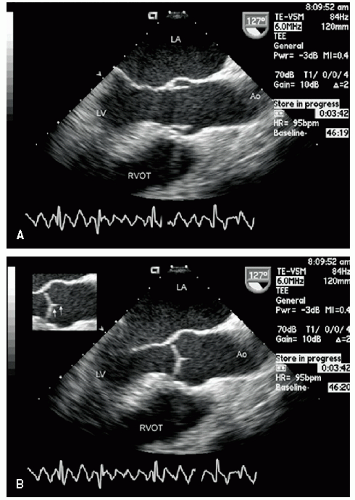
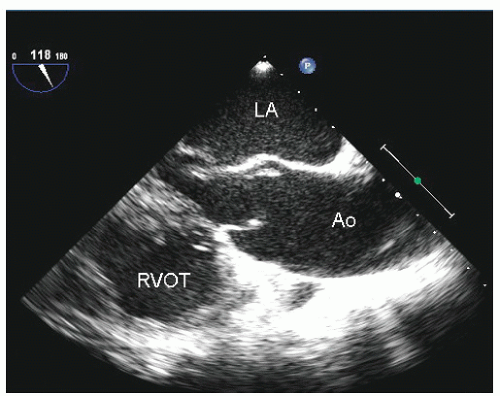
Figure 21.3 is a parasternal long-axis view of the heart with superior angulation that emphasizes visualization of the normal ascending aorta. Note the relative dimensions of the annulus, sinuses, sinotubular junction, and ascending aorta, which can be accurately determined from this transthoracic image. The suprasternal notch provides an additional window for visualization of the arch and great vessels of the aorta.
Figure 21.4 was recorded in a normal individual in whom the majority of the arch and great vessels can be easily visualized. Imaging from the suprasternal view often is more feasible in children and adolescents than in adult patients. The examiner should be aware that placement of the ultrasound
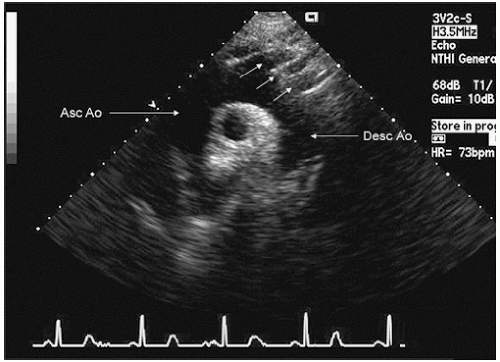
probe in the suprasternal notch may result in mild patient discomfort. Finally, transthoracic echocardiography can visualize a limited portion of the descending thoracic aorta (
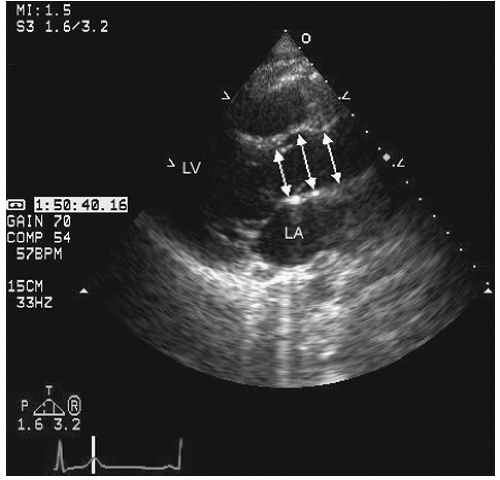
Fig. 21.5). In the parasternal long-axis view, the descending thoracic aorta appears as a circular structure behind the left atrium. On occasion, it can be confused with a dilated coronary sinus; however, the proximity of the coronary sinus to the atrioventricular groove as well as the more rigid shape of the aorta should be accurate discriminating features.
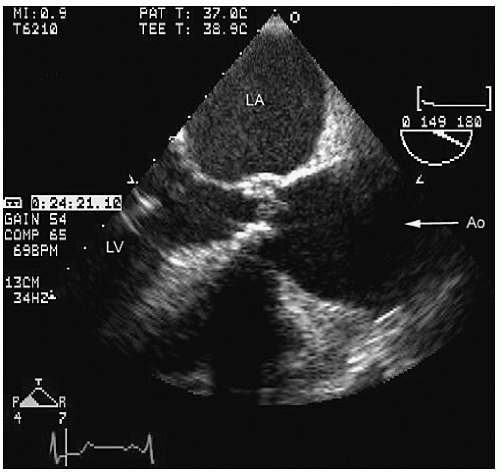
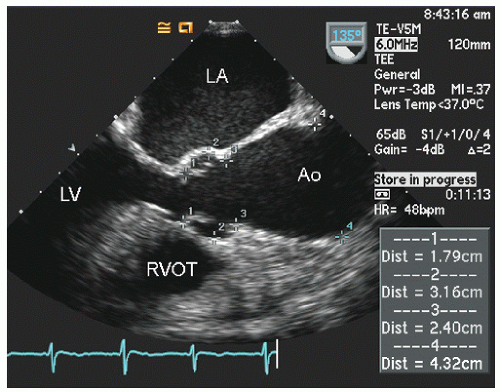
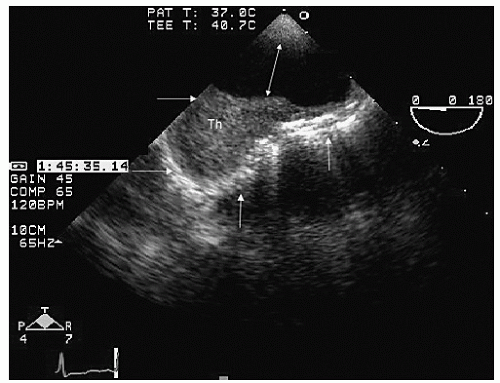
Transesophageal echocardiography provides a substantially broader window to aortic anatomy. The aorta can be visualized from the annulus through the ascending aorta, arch, and descending thoracic aorta to the level of the gastroesophageal junction.
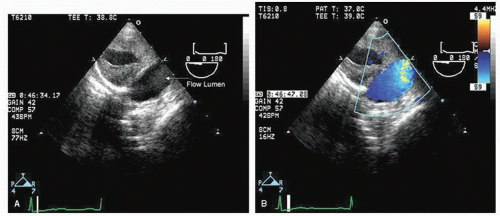
Figures 21.6,
21.7,
21.8 and
21.9 are transesophageal echocardiographic images recorded in patients with normal thoracic aortas. The relative sizes of the annulus, sinuses, sinotubular junction, and proximal ascending aorta can be appreciated in
Figure 21.6. By imaging at a 40° to 60° plane, the three sinuses and aortic cusps can be visualized simultaneously (
Fig. 21.7). The arch and descending portions of the aorta can be easily appreciated as well (
Figs. 21.8 and
21.9).
Typically, transesophageal echocardiographic imaging of the aorta begins with imaging of the ascending aorta with the probe behind the left atrium. Generally, the proximal 5 to 10 cm of the ascending aorta can be visualized by scanning at a 120° imaging plane. By rotating the imaging plane to a 40° to 60° view, a series of short-axis views of the proximal ascending aorta can be obtained, including a short-axis view of aortic valve closure (
Fig. 21.7). The descending thoracic aorta is imaged by inserting the probe deeper toward the gastroesophageal junction, rotating it 180° to face posteriorly and scanning at a 0° imaging plane. The probe can then be slowly withdrawn along the length of the aorta and a continuous series of short-axis views of the thoracic aorta obtained (
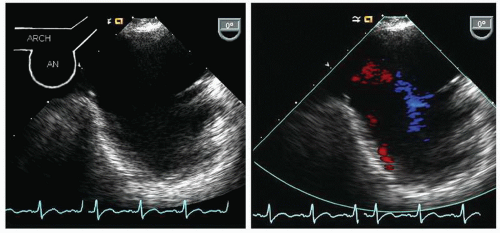
Fig. 21.8). At any point along the course of the aorta, the image can be rotated to a 90° plane for a longitudinal view of the aorta. In elderly patients, the aorta becomes tortuous, and rotation of the probe is frequently necessary to maintain a short-axis view of the aorta in the center of the imaging plane. When visualizing the arch, it should be emphasized that the probe will be at a relatively shallow depth (15-25 cm from the incisors), which results in a more dramatic curvature of the probe in the oropharynx which is less well tolerated than are deeper probe positions. The arch is best visualized by slowly withdrawing the probe to the level of the left subclavian artery, and as the probe is further withdrawn, it is rotated clockwise to obtain an elongated view of the arch (
Fig. 21.9). At the point that the arch is seen at the apex of the scanning plane, the multiplane probe can be rotated to a 90° imaging plane and a short-axis view of the apex of the arch can be recorded. By rotating clockwise and counterclockwise, the takeoff of the great vessels can often be visualized from this view.
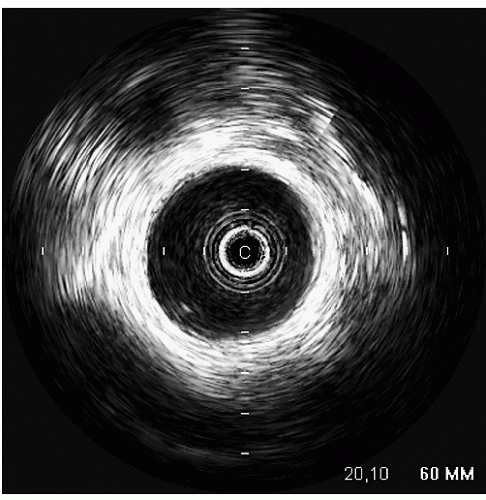
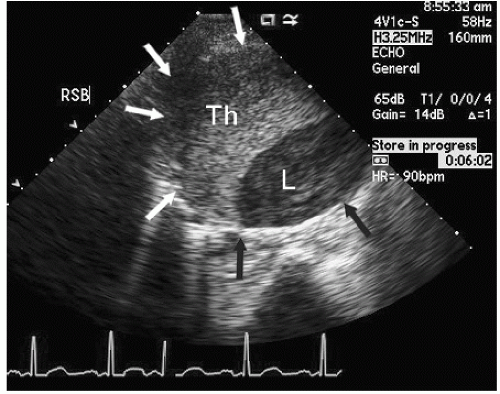
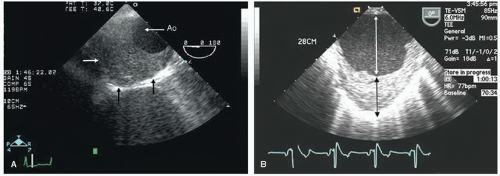
An additional ultrasound modality that has been used in evaluation of the aorta is intravascular ultrasound (
Fig. 21.10). This can be performed with high-frequency (20-30 MHz) transducers or more recently with an intracardiac probe operating
at 5.5 to 10 MHz. These higher-frequency probes provide a highly detailed, high-resolution view of intraaortic anatomy including visualization of the intimal and medial layers when using the higher-frequency probes. Intravascular ultrasound has been used in the diagnosis and management of aortic dissection and as a primary imaging tool to monitor therapeutic fenestration performed for acute aortic dissection. Intravascular ultrasound has the advantage of being able to image the entire aorta, from the root to the iliac artery. It clearly demonstrates the true and false lumens, the dissection flap, and thrombosed false lumen. It can also demonstrate the origin of each of the abdominal aortic branches (iliac artery, mesenteric branches, renal arteries), detecting whether they arise from true or false lumen. Intimal tear sites can also be imaged. Determination of aortic segment dimensions by this technique correlate precisely with computed tomographic and transesophageal echocardiographic measurements.
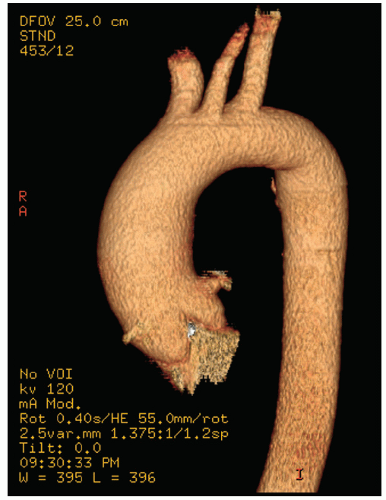
Both CT and MRI can play a valuable role in defining the anatomy of the aorta. They have the benefit of high-resolution imaging of the entire extent of the aorta from its origin at the annulus to the bifurcation of the femoral arteries. Modern CT and MRI techniques allow three-dimensional reformatting, which provides excellent spatial representation of aortic aneurysm and pseudoaneurysm. In addition, they can provide a high-resolution view of the internal lumen of the aorta with respect to penetrating ulcer and atherosclerotic involvement. They also have an advantage compared with echocardiographic techniques in the visualization of branch vessels.
Aortic Dilation and Aneurysm
Dilation of the aorta can occur at any point along its course. It is important to recognize that identification of disease in one portion of the aorta should prompt evaluation of the full extent of the aorta because many diseases affecting one portion of the aorta can also have manifestations in other areas as a part of a generalized aortopathy. An aneurysm is defined as enlargement to more than 1.5 times the normal dimension for that aspect of the aortic anatomy. Dilation of the aorta can either be isolated or associated with other cardiovascular diseases such as hypertension or aortic valve disease. It has become well established that a bicuspid aortic valve may often be associated with concurrent primary disease of the aorta. Idiopathic dilation and tortuosity have often been referred to as annuloaortic ectasia. It is unclear whether this is a distinct disease entity or related to the effects of aging, hypertension, or unrecognized primary disease of the aorta. Primary aortic dilation occurs with cystic medial necrosis, as typified by Marfan syndrome, but is also seen in other connective tissue diseases. Cystic medial necrosis is also seen as a secondary feature of many other forms of chronic aortic pathology and is not pathognomonic of a specific disease entity. This process results in weakening of the medial layers with subsequent dilation and aneurysm formation of the aorta. When associated with Marfan syndrome, it characteristically involves the ascending aorta and sinuses. Secondary dilation of the aorta can occur in volume or pressure overload states such as aortic insufficiency or hypertension. “Poststenotic” aortic dilation occurs in patients with valvular aortic stenosis and probably represents concurrent primary disease of the aorta, rather than being secondary to any specific hemodynamic abnormality (
Fig. 21.11).
Dilation of the proximal ascending aorta can often be appreciated from the transthoracic parasternal long-axis view. As noted previously, the aortic annulus is a relatively stable structure that does not dilate to any significant degree. Dilation of the proximal aorta is far more common. In
Figures 21.12,
21.13,
21.14 and
21.15, note the variable degree of aortic dilation as it extends from the annulus to the ascending aorta. There has been effacement, or loss of tapering at the sinotubular junction. Because the aortic valve cusps insert circumferentially along the sinotubular junction, dilation or effacement at this level results in malcoaptation and secondary aortic insufficiency (
Fig. 21.12).
Aneurysms in the ascending aorta, which occur past the sinotubular junction are better visualized with transesophageal
echocardiography.
Figures 21.16,
21.17 and
21.18 were recorded in patients with ascending aortic aneurysms. Note the fairly broad range of both dilation and asymmetry that can be seen.
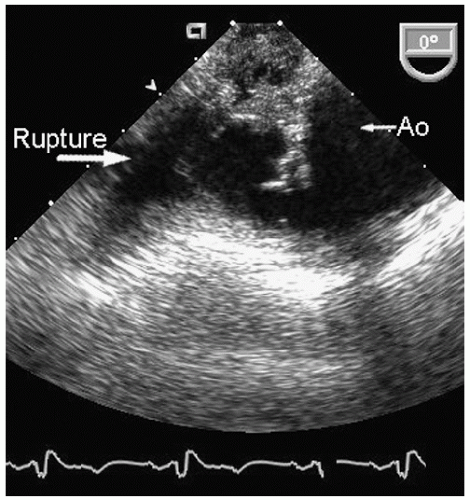
For patients with dilation or aneurysm of the ascending aorta, the likelihood of rupture or spontaneous dissection is directly related to the degree of dilation. Currently, a threshold of 55 mm is considered an indication for prophylactic aortic surgery in an effort to reduce the likelihood of a catastrophic event such as rupture or dissection. In addition, a rapid change in the degree of dilation, usually defined as more than 5 mm per year is often used as an indication for surgery. As operation success and outcomes have improved, many centers are using a threshold of 50 mm as an indication for surgery. It is not unreasonable to adjust this threshold based on gender or body size; however, firm guidelines regarding adjustment have not been established.
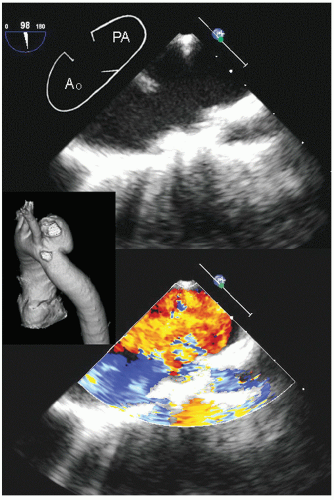
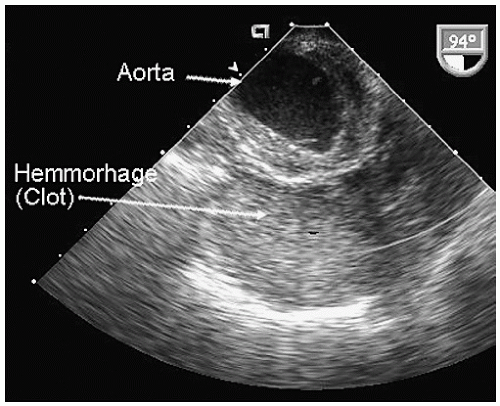
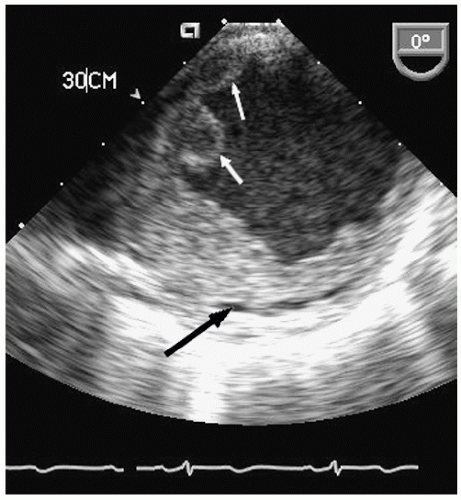
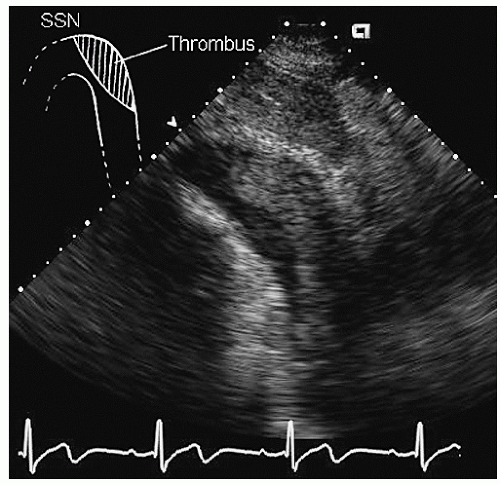
Aneurysms of the arch and descending thoracic aorta can also be accurately diagnosed and followed using transesophageal echocardiography. Aneurysms of the descending thoracic aorta frequently coexist with substantial atherosclerotic involvement, which can include protruding and mobile components as well as laminar thrombus. Chronic descending aortic aneurysms may be associated with chronic dissection.
Figures 21.19,
21.20,
21.21,
21.22,
21.23,
21.24,
21.25,
21.26,
21.27 and
21.28 were recorded in patients with arch and descending thoracic aortic aneurysms and depict
recognized complications. Because of more limited imaging planes, aneurysms of the arch may be more difficult to fully visualize and CT or MRI should be considered for full characterization, including assessment of the takeoff of the great vessels. The same considerations regarding size and likelihood of rupture and need for prophylactic surgical repair pertain to the descending thoracic aorta as for the ascending thoracic.
Marfan Syndrome
Marfan syndrome is a heritable disorder of connective tissue that is associated with characteristic cardiac abnormalities. These include dilation of the ascending aorta. The sinuses often are disproportionately involved and early cases may have
only mild dilation of the sinuses of Valsalva.
Figures 21.29,
21.30,
21.31 and
21.32 were recorded in individuals with Marfan syndrome and proximal aortic involvement. The range of aortic dilation can be relatively mild, as seen in
Figure 21.29 (left), or massive, as seen in
Figures 21.30 and
21.31. Aortic insufficiency occurs in Marfan syndrome due to dilation of the sinotubular junction, which results in loss of normal aortic cusp coaptation.
Figure 21.32 was recorded in a patient with significant aortic insufficiency due to sinotubular dilation and malcoaptation of the aortic cusps.
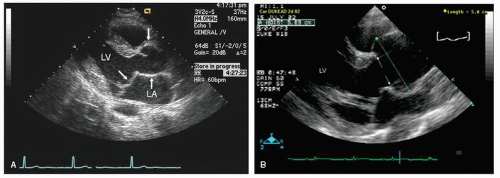
Typically, transthoracic echocardiography suffices for monitoring the size and change in proximal aortic dimensions. Once aortic abnormalities have been noted in a patient with suspected Marfan syndrome, it is important to further characterize cardiac anatomy because there is a high prevalence of mitral valve prolapse as well (
Fig. 21.29). Because of its noninvasive nature, transthoracic echocardiography should be considered the initial screening tool for patients or first-degree
relatives with suspected Marfan syndrome, and transesophageal echocardiography should be reserved only for further specific characterization. In patients with Marfan syndrome any portion of the aorta may be involved, and CT or MRI should be considered for comprehensive screening in selected cases. One identified as having aortic dilation, routine (probably annual) reexamination is indicated to assess for progression of disease.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access