Bioprosthetic valve thrombosis is uncommon and the diagnosis is often elusive and may be confused with valve degeneration. We report our experience with mitral bioprosthetic valve thrombosis and suggest a therapeutic approach. From 2002 to 2011, 149 consecutive patients who underwent mitral valve replacement with a bioprosthesis at a single center were retrospectively screened for clinical or echocardiographic evidence of valve malfunction. Nine were found to have valve thrombus. All 9 patients had their native valve preserved, representing 24% of those with preserved native valves. Five patients (group 1) presented with symptoms of congestive heart failure at 16.4 ± 12.4 months after surgery. Echocardiogram revealed homogenous echo-dense film on the ventricular surface of the bioprosthesis with elevated transvalvular gradient, resembling early degeneration. The first 2 patients underwent reoperation: valve thrombus was found and confirmed by histologic examination. Based on these, the subsequent 3 patients received anticoagulation treatment with complete thrombus resolution: mean mitral gradient decreased from 23 ± 4 to 6 ± 1 mm Hg and tricuspid regurgitation gradient decreased from 83 ± 20 to 49 ± 5 mm Hg. Four patients (group 2) were asymptomatic, but routine echocardiogram showed a discrete mass on the ventricular aspect of the valve: 1 underwent reoperation to replace the valve and 3 received anticoagulation with complete resolution of the echocardiographic findings. In conclusion, bioprosthetic mitral thrombosis occurs in about 6% of cases. In our experience, onset is early, before anticipated valve degeneration. Clinical awareness followed by an initial trial with anticoagulation is warranted. Surgery should be reserved for those who are not responsive or patients in whom the hemodynamic status does not allow delay. Nonresection of the native valve at the initial operation may play a role in the origin of this entity.
Bioprosthetic valves are usually implanted in patients for whom warfarin poses an increased risk for bleeding. Although these valves have a low propensity for thrombosis, there is still an annual risk of approximately 0.03%. The cause of bioprosthetic valve malfunction is usually confirmed after surgical removal or at autopsy. Preoperative diagnosis of valve thrombosis has seldom been reported. During the last 10 years, we encountered a number of patients who had undergone mitral valve replacement with a bioprosthesis presenting with early bioprosthetic valve malfunction caused by thrombus deposition on the valve. We present the clinical and echocardiographic features of mitral bioprosthetic valve thrombosis and our recommended therapeutic approach.
Methods
A retrospective query of our database identified 149 consecutive operative survivors who had undergone mitral valve replacement with a bioprosthesis from 2002 to 2011. Patient clinical and echocardiographic data were reviewed to detect evidence of valve malfunction. The present study was approved by the Institutional Ethics Committee that waived the need for patient consent. Clinical characteristics and echocardiographic data in patients who developed valve thrombus and those who did not are listed in Table 1 . Patients with thrombus were grouped according to the clinical presentation.
| Variable | Valve Thrombus, n = 9 (%) | Control, n = 140 (%) | p |
|---|---|---|---|
| Age (yrs) | 73 ± 7 | 69 ± 10 | 0.2 |
| Men | 7 (78) | 73 (52) | 0.1 |
| Hypertension | 8 (89) | 109 (78) | 0.4 |
| Diabetes mellitus | 2 (22) | 44 (31) | 0.6 |
| Renal failure | 1 (11) | 29 (21) | 0.5 |
| Pulmonary hypertension | 8 (89) | 110 (79) | 0.4 |
| Previous MI | 3 (33) | 36 (26) | 0.4 |
| Atrial fibrillation | 2 (22) | 54 (39) | 0.3 |
| NYHA class III–IV | 7 (78) | 121 (86) | 0.3 |
| EuroSCORE logistic | 11 ± 6 | 16 ± 16 | 0.1 |
| LV dysfunction | 2 (22) | 29 (21) | 0.6 |
| LVEDD (mm) | 57 ± 7 | 53 ± 9 | 0.2 |
| LVESD (mm) | 37 ± 9 | 35 ± 1 | 0.5 |
| MR (moderate to severe) | 8 (89) | 127 (91) | 0.6 |
| MS (severe) | 3 (33) | 43 (31) | 0.6 |
| TR gradient (mm Hg) | 49 ± 11 | 48 ± 19 | 0.8 |
| Implanted valve size (mm) | 25.2 ± 3 | 26.6 ± 1.7 | 0.2 |
| Nonresection of native valve | 9 (100) | 36 (24) | <0.0001 |
At surgery, the native valve was preserved in its entirety in 37 patients (25%). The bioprosthesis was implanted using interrupted 2-0 braided polyester mattress sutures reinforced with pledgets on the atrial side of the annulus. The anterior leaflet was split and plicated toward the annulus, with the stitches used for securing the bioprosthesis. A porcine bioprosthesis was implanted in 143 patients: Hancock II (Medtronic Inc., Minneapolis, Minnesota) in 139 and St. Jude Epic (St. Jude Medical, Minneapolis, Minnesota) in 4. A pericardial valve was implanted in 6 patients (Carpentier Edwards; Edwards Lifesciences, Irvine, California). The valves were rinsed as per the manufacturer protocol. All patients received low-molecular-weight heparin starting the day after surgery, followed by warfarin for 3 months with a target international normalized ratio at around 2.5. All operative procedures were performed by the same surgical team using the same surgical technique.
Postoperative echocardiographies were routinely performed before discharge; 1, 6, and 12 months after surgery; and annually thereafter. Standard 2-dimensional (2D) and 2D-guided M-mode left atrial and left ventricular dimensions were obtained from images obtained in left parasternal views. Leaflet mobility and thickness were analyzed from multiple views. Qualitative changes in cusp thickness and mobility, and the presence and characteristics of left atrial and valvular masses, were assessed from both transthoracic echocardiographic and transesophageal echocardiographic images. Mitral valve gradient and regurgitation were evaluated by color and continuous-wave Doppler according to previously established methods.
Chi-square univariate analysis was used to compare clinical and echocardiographic characteristics between patients who did and those who did not develop prosthetic valve thrombosis. Multivariate regression analysis was used to identify predictors for the development of valve thrombosis.
Results
Overall, 10 patients were diagnosed with bioprosthetic mitral valve malfunction. One underwent surgery and pannus formation was found to be the cause; this patient was excluded from this report. Prosthetic valve thrombus was diagnosed in 9 (6%). The interval between surgery and diagnosis was 11.9 ± 10.6 months (range 2 to 37).
Five patients (group 1) presented with symptoms of congestive heart failure. All 5 showed clinical and echocardiographic signs of mitral stenosis, with high mitral valve gradients and elevated pulmonary pressure. In these patients, transthoracic echocardiogram revealed marked bioprosthetic leaflet thickening. The mean mitral gradient was 22 ± 3.2 mm Hg (range 19 to 27) and tricuspid regurgitation (TR) gradient was 71 ± 21 mm Hg. Left atrial or left atrial appendage thrombi were not detected. The bioprosthetic valve leaflets appeared thickened because of homogenous echo-dense masses on the ventricular surface, with restricted leaflet opening. In 1 patient, there was severe mitral regurgitation with a central jet. The interval between surgery and diagnosis was 16.4 ± 12.4 months (range 6 to 37). The diagnosis of valve thrombus was made at repeat surgery in 2 and after a successful trial of anticoagulation in 3.
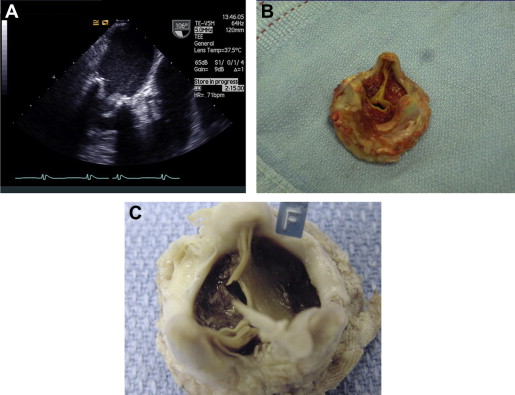
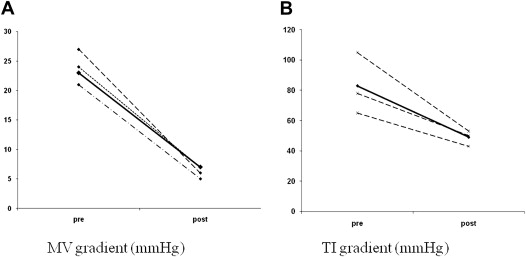
The first 2 patients underwent reoperation, replacing the bioprosthesis with a mechanical valve. In both, we found a macroscopic layer of thrombus on the ventricular aspect of the mitral valve leaflets ( Figure 1 ). One died because of sepsis with multiorgan failure on the twenty-ninth postoperative day. In these 2 patients, the diagnosis of valve thrombosis was made at pathologic examination and not preoperatively. In the subsequent 3 group 1 patients, we suspected that the cause of valve malfunction was thrombosis; therefore, they were treated with anticoagulation. Initial treatment consisted of full anticoagulation with intravenous heparin until clinical and echocardiographic improvement was observed, followed by warfarin for a target international normalized ratio of 3.0 to 3.5 for life. Clinical improvement and a marked reduction in transvalvular gradient, coupled with a reduction in pulmonary pressure, were evident within 3 to 6 days. Complete thrombus resolution was observed in echocardiographies performed after 9 to 75 days. Of note, in those patients who were hospitalized, echocardiographic resolution was seen by 20 days ( Figure 2 ). In 1 patient, follow-up transthoracic echocardiography was performed only after 75 days. It seems reasonable to assume that the thrombus had disappeared much earlier. Mitral regurgitation, which was present in 1 patient, disappeared almost entirely by day 7 of treatment. In all 3 patients treated with anticoagulation, serial echocardiograms showed a reduction in transmitral valvular gradients coupled with a reduction in the TR gradient: the mean mitral gradient decreased from 23 ± 4 to 6 ± 1 mm Hg and the TR gradient from 83 ± 20 to 49 ± 5 mm Hg ( Figure 3 ).
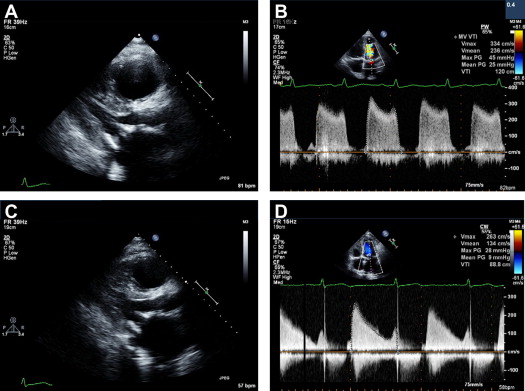
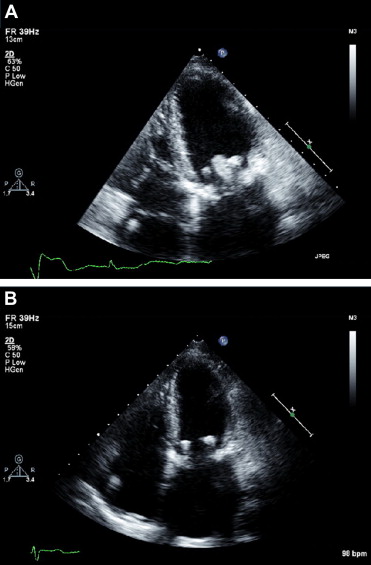
In 4 additional patients (group 2), routine transthoracic echocardiogram revealed a discrete sessile mass attached to the ventricular aspect of the bioprosthesis at 6 ± 4.3 months (range 2 to 12) after surgery. One underwent a repeat surgery to replace the valve at another center, and histology showed the mass to be a thrombus. Three were treated successfully with anticoagulation, with complete resolution of the echocardiographic findings ( Figure 4 ). Of note, these patients were asymptomatic, and the findings were detected on routine follow-up echocardiograms.
There was no clinical or laboratory evidence of endocarditis in any of the 9 patients. In all patients who developed valve thrombosis, the native valve had been preserved in its entirety, so that in effect 9 of 37 patients (24%) with a nonresected native valve developed thrombosis. In those patients who underwent reoperation, no thrombus was found in the left atrium or the left atrial appendage, and all were confirmed by histologic examination of the explanted valves, with no evidence of infection. Multivariate stepwise regression showed nonresection of the native mitral valve to be the only predictor for development of valve thrombosis (p = 0.01). Atrial fibrillation, gender, origin of mitral valve disease, and implanted valve size or type were not associated with development of thrombus. There was no history of thromboembolism in any of the cases that developed valve thrombus. At the time of preparation of this manuscript, there was no case of recurrent thrombosis of which we were aware. A clinical summary of the patients who developed thrombus is listed in Table 2 .



