Descending Thoracic and Thoracoabdominal Aortic Aneurysms
Matthew L. Stone
John A. Kern
INTRODUCTION
Aortic aneurysms are defined as a doubling of the normal aortic diameter for a particular body surface area, age, and gender. Advancements in open surgical technique and the advent of endovascular treatment have supported a significant improvement in outcomes and survival for many with descending thoracic and thoracoabdominal aortic aneurysms. Since the inception of successful thoracic and thoracoabdominal aortic reconstruction by Etheridge and DeBakey in 1955 and 1956, commitment to technical refinement and risk stratification has expanded the treatment potential for patients with thoracoabdominal aortic aneurysmal disease. The promise of current treatment has introduced preventative standards for reconstruction in the setting of chronic aortic aneurysm while advancing the success of treatment for acute aortic rupture and malperfusion. A principle achievement over the past decade of care has been the standardization of operative and critical care techniques for the maintenance of branch vessel and end-organ perfusion, which have minimized rates of postoperative organ failure and paraplegia. As we embark on a promising future for open and endovascular repair techniques, a continued commitment to evidence-based practice is imperative to further the curative potential for patients with descending thoracic and thoracoabdominal aortic disease.
The focus of this chapter is to review the current diagnostic standards and operative principles for the surgical treatment of thoracic and thoracoabdominal aortic aneurysmal disease. Open and endovascular techniques are presented as both independent and hybrid approaches to thoracic and thoracoabdominal aortic reconstruction.
ANATOMIC PRINCIPLES
Descending thoracic aortic aneurysms arise in the thoracic aorta distal to the origin of the left subclavian artery. Thoracoabdominal aortic aneurysms span the diaphragmatic hiatus at the level of T12 and introduce important considerations for pleural entry, peritoneal access, and diaphragmatic conservation for aortic repair. In addition, phrenic nerve localization and preservation are imperative within the pericardium as it crosses the left atrium and terminates distally on the abdominal surface of the diaphragm.
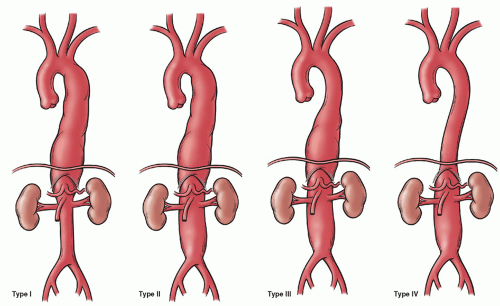
Thoracoabdominal aortic aneurysms may be classified according to the anatomic extent of disease as proposed by the Crawford classification system: type (or extent) I (24%) involving the proximal descending thoracic to proximal abdominal aorta, type II (26%) involving the proximal descending thoracic to infrarenal abdominal aorta, type III (26%) involving the distal descending thoracic and abdominal aorta, and type IV (24%) involving the abdominal aorta and including visceral vessel segments (Fig. 59.1). An understanding of the visceral and somatic branches along the thoracoabdominal aorta provides the foundation for operative techniques that maintain spinal cord and organ perfusion. Paired somatic branches arise from the third intercostal arteries and continue to the fourth lumbar arteries. The critical vascular zone of the spinal cord from T4 to L1 vertebral levels is characterized by the least prominent blood supply and is the zone at which interference with the circulation is most likely to result in paraplegia. Importantly, this anatomic guideline may vary based on the presence and extent of preexisting aortic reconstruction. The artery of Adamkiewicz is the largest anterior medullary feeder for the supply of the lumbar cord and arises from the lower intercostal or lumbar artery on left in 65% to 80% of cases between T6 and L4 levels. The lower intercostal and lumbar artery supplying the artery of Adamkiewicz should be preserved or reconstructed to maintain blood supply to the lumbar spinal cord to minimize the risk of spinal cord injury. Perfusion through medullary arteries maintains spinal cord blood supply proximal to the aortic cross-clamp and determines postoperative neurologic function. Visceral branches are located on the ventral aorta as the celiac axis, superior mesenteric, inferior mesenteric, and paired or multiple renal arteries.
Localization of somatic and visceral branches during each individual aortic reconstruction guides decisions regarding clamp placement, perfusion techniques, and the need for reimplantation or graft reconstruction. Multidetector row computed tomography (CT) provides characterization of the critical spinal cord blood supply for targeted reconstruction and may be considered for elective complex repairs. The anterior spinal artery is a principal component of the extensive intraspinal and paraspinous collateral blood supply to the anterior spinal cord, with 75% of all segmental arteries providing direct anterior spinal artery-supplying branches. This extensive collateral network imparts a responsibility to define dominant intercostal and lumbar arteries for each individual patient to guide operative planning for reconstruction and preservation of blood supply to the spinal cord.
EPIDEMIOLOGIC AND DIAGNOSTIC CONSIDERATIONS
The prevalence of thoracic aortic aneurysms has tripled over the past two decades to a current estimate of 10.4 cases per 100,000 person-years. Patients with thoracic aortic aneurysms are at a mean age of 59 to 69 years with a 2:1 to 4:1 male predominance. Unlike ascending aortic aneurysms, descending aneurysms are often associated with significant atherosclerosis, yet this may represent more of an association than causal relation. The natural history of aortic aneurysm formation is diverse and may
result from cystic medial necrosis, chronic dissections, aortitis, traumatic transaction, or conditions related to the degeneration of the aortic media such as Marfan syndrome, Ehlers–Danlos, annuloaortic ectasia, trauma, infections, mycotic conditions, syphilis, or idiopathic causes. Hypertension predisposes a patient to aortic dissection and subsequent potential aneurysm formation secondary to the increased thrombogenicity and incomplete decompression of the aortic false lumen. In retrospective review, the incidence of degenerative aneurysms without dissection was 73%, concomitant chronic dissection was 23%, and acute aortic dissection was 4%.
result from cystic medial necrosis, chronic dissections, aortitis, traumatic transaction, or conditions related to the degeneration of the aortic media such as Marfan syndrome, Ehlers–Danlos, annuloaortic ectasia, trauma, infections, mycotic conditions, syphilis, or idiopathic causes. Hypertension predisposes a patient to aortic dissection and subsequent potential aneurysm formation secondary to the increased thrombogenicity and incomplete decompression of the aortic false lumen. In retrospective review, the incidence of degenerative aneurysms without dissection was 73%, concomitant chronic dissection was 23%, and acute aortic dissection was 4%.
History and physical examination remain principal tenants to the management of a patient with presumptive descending thoracic and thoracoabdominal aortic aneurysm. The primary aim of physical examination is to exclude acute dissection, rupture, and malperfusion. Neurologic deficits, acute abdominal pain, hematuria, and lower extremity ischemia are indications of aneurysm-related malperfusion or distal embolism and prompt immediate intervention. Hypotension with hemorrhage into the left chest and pericardium represent rupture and support emergent resuscitation and repair. Acute or chronic enlargement of the thoracic aorta may manifest as chest and back pain or hoarseness secondary to recurrent laryngeal nerve compression. A nonproductive cough or hemoptysis may represent contained rupture or bronchial irritation, while gastrointestinal hemorrhage may occur in the setting of an aortoenteric fistula.
While an estimated 65% of patients with thoracic aortic aneurysms are symptomatic at the time of operative intervention, an increasing number of asymptomatic aneurysms are now detected on unrelated imaging. Primary risk factors for the development and size progression of thoracic aortic aneurysm include hypertension, smoking, and chronic obstructive pulmonary disease. Aortic wall tension and associated risk of rupture increase as the aneurysmal segment increases in diameter, as represented by Laplace’s law. The descending thoracic aorta is estimated to grow on average 0.19 cm per year and can attain a growth rate as high as 0.28 to 0.48 cm per year in the presence of aortic dissection.
IMAGING
The choice of imaging technique for evaluation of the thoracic aorta is determined by patient-related factors (hemodynamic stability, renal function, contrast allergy) and institutional capabilities (technologic capability, expertise). CT-induced contrast nephropathy and magnetic resonance (MR)-associated gadolinium nephrogenic systemic fibrosis are principal considerations for patients with borderline kidney function (serum creatinine >1.8 to 2.0 mg/dl).
Chest X-ray has demonstrated specificity for acute aortic pathology of 86% in prospective study of patients undergoing evaluation for acute thoracic aortic disease. This imaging modality is, however, insufficient to definitely exclude thoracic aortic aneurysm in high-risk patients and lacks anatomic detail necessary for directed treatment. CT angiography (CTA) is accepted as the primary diagnostic imaging modality for the thoracic aorta with a demonstrated accuracy of 92% for all-inclusive abnormalities of the thoracic aorta and has an established efficacy in the prediction of the need for hypothermic circulatory arrest during surgical repair. Image acquisition should include thoracic branch vessels and femoral vessels to determine aneurysm extension and to guide potential endovascular access. Three-dimensional CTA reconstruction may provide additional advantages to operative planning.
MR angiography (MRA) provides similar advantages to CTA without the limitations of radiation exposure or iodinated contrast. Phase-contrast techniques and two-dimensional time-of-flight modalities have increased the application of MRA in the setting of thoracic aortic aneurysm and dissection, with beneficial applications in the determination of flow dynamics within the false channel. First-line adoption of this imaging modality in the setting of thoracic aortic aneurysm remains limited by institutional capabilities and the time required for acquisition. Current aortic sizing for intervention and endograft sizing are based on the external aortic diameter derived from CTA or MRA or the internal aortic diameter on echocardiography. In addition to providing insight regarding aortic size and anatomic aneurysm characteristics, both CTA and MRA guide the selection of safe sites for arterial cannulation and cross-clamp application.
The overall risk of rupture at 5 years following the initial diagnosis of descending thoracic or thoracoabdominal aortic aneurysm is estimated to be 20% and is dependent on the aortic size at diagnosis: 0% for aneurysms <4 cm diameter, 16% for those 3 to 5.9 cm, and 31% for aneurysms 6 cm or more. In addition, yearly composite adverse outcomes of rupture, dissection, and death are estimated to occur in 14.1% of patients with aneurysms 6 cm and greater in diameter in the absence of surgical intervention. These data support size-related treatment as represented by the expert American Heart Association consensus guidelines for intervention based on Class I evidence:
Open repair is recommended for chronic descending thoracic aortic dissections with an aortic diameter exceeding 5.5 cm.
Endovascular repair should be considered for degenerative aneurysms exceeding 5.5 cm, saccular aneurysms, and postoperative pseudoaneurysms.
Elective surgery is recommended for thoracoabdominal aneurysms exceeding 6.0 cm or less in the setting of connective tissue disease with limited endovascular options and elevated surgical morbidity.
Additional revascularization procedures are recommended for patients with end-organ ischemia or visceral artery atherosclerotic disease.
Patients with symptoms consistent with thoracic aneurysm enlargement warrant prompt surgical intervention unless candidacy is limited by comorbid disease or quality of life.
For endovascular techniques, a suitable landing zone of normal diameter with no circumferential thrombus 2 to 3 cm distal to the left subclavian or left common carotid artery and proximal to the celiac axis is required. For patients with anatomic limitations, endovascular techniques may also create landing zones for candidate aneurysms. In addition, a femoral vessel diameter > 7 mm and an aortic curvature <60 degrees optimize sheath advancement and stent deployment. Endograft sizing recommendations are to aim for 10% to 20% over sizing at the proximal landing zone. These guidelines represent current suggested thresholds within the rapidly advancing field of endovascular aortic aneurysm repair and should not be accepted as absolute criteria. With both endovascular and open repairs, the decision for surgical intervention and reconstruction is founded on a calculated heightened risk of nonoperative medical management that exceeds the risk of the selected operation. A preoperative understanding of potential ischemic heart disease, obstructive lung disease, and renal impairment is imperative prior to operative intervention. Medical management and optimization of end-organ function prior to endovascular or open repair is a critical consideration for all patients to minimize perioperative morbidity and mortality.
OPEN DESCENDING AND THORACOABDOMINAL RECONSTRUCTION
Anesthesia and Monitoring
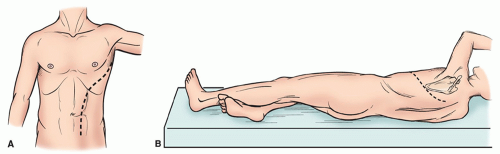
Induction and maintenance of general anesthesia are achieved by double-lumen endotracheal intubation. A single-lumen endotracheal tube may be utilized for aneurysms isolated to the lower thoracic and upper abdominal aorta. Large-bore peripheral access, central venous pressure monitoring, continuous arterial pressure assessment, pulse oximetry, and foley catheter are imperative to guide intraoperative resuscitation and support. Transesophageal echocardiography, continuous electrocardiographic monitoring, and a pulmonary artery catheter provide additional adjuncts for intraoperative cardiac evaluation and are routine at our institution. Femoral artery access is needed with cardiopulmonary bypass to maintain balanced pressures. Temperature monitoring is achieved at two access sites to estimate cerebral (blood, esophageal, tympanic membrane, nasopharynx) and visceral (bladder, rectal) temperatures. Cerebrospinal fluid (CSF) drainage is the principal modality for spinal cord protection utilized at our institution due to the established benefit and low-risk of this protective strategy. Motor-evoked potential (MEP) and somatosensoryevoked potential (SSEP) monitoring may be applied in addition to CSF drainage for spinal cord protection and monitoring. Following anesthesia induction, the patient is rolled to expose the left flank and chest wall with the pelvis rolled half-anteriorly to enable access to the left femoral vessels (Fig. 59.2). The table is flexed to open the intercostal spaces, axillary rolls are applied, and pressure points are padded with care.
Incision and Exposure: Thoracic Aneurysms
An extended posterolateral thoracotomy is performed to expose the entire length of the thoracic aorta. The latissimus dorsi and a minimal portion of the serratus anterior muscles are divided. Left lung ventilation is terminated prior to entry into the pleural cavity. Aneurysms of the upper or middle thoracic aorta are accessed through a single intercostal space, which may be facilitated by division of one of the ribs posteriorly. We prefer two separate sites of entry through the fourth interspace and the seventh or eighth interspace for more extensive descending thoracoabdominal aneurysms. The fourth interspace is critical to proximal clamp application. Careful entry into the pleura allows anterior retraction of the lung under sponges to expose the aorta. The pleura over the aorta is divided. The intrathoracic course of the vagus nerve, recurrent laryngeal nerve, and proximal phrenic nerve at the aortic arch is identified. The aorta is then taped immediately proximal to or at the origin of the left subclavian artery where careful, sharp dissection allows for future application of the aortic cross-clamp. The thoracic duct and adjacent lymph vessels should be avoided. Intraoperative lymph leaks should be immediately repaired. The identification of the esophagus may be augmented by the direct palpation of the nasogastric tube or transesophageal echocardiography scope to avoid inadvertent injury.
Following achievement of the proximal aortic dissection, attention is directed to the aorta distal to the aneurysm. In the setting of extensive thoracic aortic involvement, a second pleural entry through the separate intercostal incision may be needed. Careful sharp dissection of the distal aorta is performed to achieve adequate control.
Importantly, no attempts are made in the dissection of the aneurysm along its entire length to avoid potentially hazardous bleeding; however, posterior mobilization of the aneurysmal segment may be necessary to support clip application to the intercostals prior to opening of the aorta.
Importantly, no attempts are made in the dissection of the aneurysm along its entire length to avoid potentially hazardous bleeding; however, posterior mobilization of the aneurysmal segment may be necessary to support clip application to the intercostals prior to opening of the aorta.
Incision and Exposure: Thoracoabdominal Aneurysms
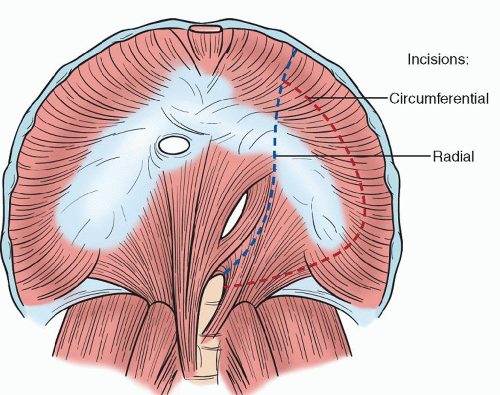
Our standard incision is started posterolaterally over the ribs of the seventh, eighth, or ninth interspace dependent on the proximal extent of the aneurysm. The incision is then advanced across the ninth interspace at the costal margin to curve inferiorly to run parallel and left-lateral to the midline and rectus sheath. Single right lung ventilation is initiated and the left pleural space is entered. The abdominal muscles are divided and the peritoneum is preserved. Special care is needed at the lateral edge of the rectus abdominis muscle where the peritoneum and transversalis fascia are closely apposed to the abdominal wall. While the retroperitoneal approach is our preferred technique, we recognize that the intraperitoneal approach affords mobilization of the spleen and left colon, which may enable better access to the abdominal aorta in select patients. The diaphragm may be taken down with a curved incision along the costal margin with care to preserve a 3-cm rim along the posterior aspect of the rib. The tendinous center of the diaphragm is then conserved when anatomically feasible to improve postoperative respiratory recovery and weaning time. This limited phrenotomy technique allows passage of the graft through the natural hiatus of the diaphragm. The incision is continued down to the crura, and the left crus may be divided to expose the aorta beneath. A radial diaphragmatic incision may also be utilized and has become our preferred technique (Fig. 59.3).
Progressive retraction of the peritoneum and its contents will facilitate exposure of the retroperitoneum and a self-retaining retractor is necessary. The aorta is located medial to the iliopsoas muscle. Mobilization of the left kidney from the bed of the psoas muscle may provide additional exposure and is preferred at our institution. Importantly, this step is deferred in patients with a retroaortic left renal vein. Anterior visceral branches are identified and sharply dissected with attention to mobilization should reimplantation or bypass be required. Dissection is performed proximal and distal to the aneurysmal segment of aorta and each site is tapped in preparation for cross-clamping.
Hemodynamic Support during Aortic Cross-Clamping
Proximal aortic cross-clamp application induces a significant increase in cardiac afterload. Sudden afterload reduction following clamp release is associated with an acute relative hypovolemia and systemic hypotension. These periods
of hemodynamic change are managed through the following intraoperative strategies.
of hemodynamic change are managed through the following intraoperative strategies.
Pharmacologic Manipulation and Clamp Application Technique
Direct and cooperative communication between surgeon and anesthesiologist is imperative during periods of cross-clamp application and release. Our approach to cross-clamp application and distal aortic perfusion involves active distal aortic perfusion with femoral-to-femoral bypass for all but type IV and V aortic aneurysms, for which we minimize supraceliac clamp times with no active distal perfusion. This perfusion strategy maintains distal aortic perfusion with warm, oxygenated blood and provides the time necessary to safely perform the most complex of repairs. With this strategy, maintenance of distal aortic blood pressure is imperative and is achieved through the titration of pharmacologic agents and pump flow rates to maintain optimal perfusion. Infusion of nitroglycerine, trimethaphan, or sodium nitroprusside prior to the application of the aortic cross-clamp may be performed to lower systolic blood pressure to 70 to 80 mmHg. The anticipated acute rise in blood pressure with aortic cross-clamp application should necessitate aggressive blood pressure control until planned clamp release. Sodium bicarbonate, calcium, and rapid volume infusion may be initiated prior to unclamping to prevent acute hypotension. Vasopressor agents may also be utilized to augment these resuscitative measures to maintain blood pressure upon clamp release. Importantly, our principal focus is to carefully titrate both volume and pump flow rate to maintain target pressure, often eliminating the need for these additional interventions throughout the critical periods of clamp application and release.
Progressive clamp application and release over a period of 2 to 4 minutes may blunt the imposed physiologic insult and hemodynamic response. In assessment of end-organ ischemia, cross-clamp time is measured from the first click of clamp application until its complete release. Despite these preventative and resuscitative measures, hemodynamic instability may occur and should be anticipated with appropriate preparation.
Extracorporeal Circulation
Extracorporeal circulation support provides after load reduction and continuous end-organ perfusion during the aortic cross-clamp period. Techniques for the maintenance of extracorporeal circulation include passive aorto-aortic shunt, left atriofemoral bypass, and femorofemoral cardiopulmonary bypass.
Left Atriofemoral Bypass
Atriofemoral bypass is achieved through an inflow circuit cannula that is introduced into the left atrial appendage or inferior pulmonary vein. The left atrial inflow blood is powered through a centrifugal or roller pump and returned to a nondiseased region of femoral artery or aorta below the level of the distal clamp. Femoral-to-femoral bypass has mostly replaced this technique at our institution due to the potential for air in the circuit and the absence of a heater– cooler pump mechanism. This technique may, however, be beneficial in patients with poor hemodynamics, impaired cardiac function, or during prolonged cross-clamp times. Improvement in long-term survival with distal aortic perfusion in addition to measures for cerebral protection supports the efficacy of both techniques in the mitigation of ischemic end-organ injury.
The technique for initiation of circuit support involves exposure and taping of the left femoral artery prior to systemic heparinization. The pericardium is opened to expose the left atrial appendage and a purse string is placed. We prefer the use of the inferior pulmonary vein to avoid opening of the pericardium and potential injury to the fragile left atrial appendage. Prior to aortic cross-clamp application, heparin (100 U/kg) is administered to achieve an activated clotting time of 200 seconds. A heparin-bonded circuit provides a measure for the reduction of systemic heparin. The pump inflow cannula is placed in the left atrial appendage or inferior pulmonary vein and the pump outflow cannula is inserted in the left femoral artery to close the circuit. The pump is activated prior to clamping to achieve a high flow rate for reduction of preload prior to the significant increase in afterload imposed by clamp application. Pump flows are maintained and adjusted to achieve target pressure above and below the aortic cross-clamp of 80 to 100 mmHg. Following reconstruction, pump flow can be terminated to increase preload prior to clamp release and the associated afterload reduction.
Femorofemoral Cardiopulmonary Bypass
Femorofemoral bypass is our preferred technique for distal aortic perfusion and is especially beneficial in the setting of a heavily calcified proximal aorta. The principal benefit to this technique is the maintenance of warm, oxygenated blood perfusion to the distal aorta and branch vessels with precise control of perfusion pressure and temperature. The inclusion of cooling capabilities within the femorofemoral bypass circuit allows the achievement of circulatory arrest when necessary. Additionally, pump sucker return to the pump reservoir minimizes transfusion requirement.
The technique for circuit achievement involves femoral vein and artery exposure preferentially on the left side for cannulation following systemic heparinization. Longer activated clotting times are required secondary to the circuit components. An extended 21-F multistage femoral vein cannula is advanced from the left femoral vein toward right atrium to achieve adequate venous drainage of 1.5 to 3 L. Arterial access is achieved at the left femoral artery, left internal iliac artery, or by direct cannulation of the distal aorta with a 15- to 17-F arterial cannula. Cardiopulmonary bypass is initiated immediately prior to aortic cross-clamp application. In patients requiring circulatory arrest, the patient is cooled to 15 to 18 degrees Celsius and circulatory arrest is initiated to allow creation of the proximal anastomosis. The left ventricle should be vented upon fibrillation in the setting of aortic insufficiency. Prior to clamp release, additional volume may be needed in the reservoir to allow rapid transfusion and high pump flows upon clamp release and anticipated hypotension.
Spinal Cord Protection
The inherent threat of unavoidable temporary cord ischemia during repair and the potentially preventable lasting ischemia resulting from inadequate perioperative cord perfusion in patients with descending thoracic and thoracoabdominal aneurysms have supported significant technical advancements in spinal cord protection. Central to methods of cord protection is an understanding of the axial network and the supplying segmental, subclavian, and hypogastric arteries and the protective effect of permissive hypothermia on neural tissue. This collateral network may increase flow through alternative routes when another is reduced; however, this network may also result in steal that will result in decreased nutrient flow to the cord. Steal may occur secondary to the absence of visceral and iliac artery perfusion, during the cross-clamp period, or as a result of pharmacologically induced arteriovenous
shunting when bleeding intercostals into an excluded aortic segment. With these considerations, we have adopted the liberal use of multihead perfusion cannulae and pediatric coronary sinus catheters for the maintenance of perfusion to the visceral and subclavian aortic branches and dominant intercostal arteries, respectively (Fig. 59.4). The detection of potentially reversible delayed paraplegia may be achieved by MEP and SSEP monitoring with immediate assessment of function postoperatively. It is not our routine practice to perform MEP or SSEP monitoring. The adoption of the following provocative techniques for reduction of spinal cord ischemic-induced injury has resulted in a significant decrease in the incidence of paralysis following open repair that is dependent on the extent of repair: 15% for type I, 30% for type II, 7% for type III, and 4% for type IV. Additional predictors of delayed neurologic deficit for thoracoabdominal aneurysms include emergent operative status, prolonged aortic cross-clamp time, level of cross-clamp, hypogastric artery exclusion, aortic rupture, preoperative renal dysfunction, prior abdominal aortic aneurysm repair, acute dissection, and extent II involvement. These techniques and the avoidance of hemodynamic instability and significant blood volume loss have offset the influence of cross-clamp time on induced spinal cord injury.
shunting when bleeding intercostals into an excluded aortic segment. With these considerations, we have adopted the liberal use of multihead perfusion cannulae and pediatric coronary sinus catheters for the maintenance of perfusion to the visceral and subclavian aortic branches and dominant intercostal arteries, respectively (Fig. 59.4). The detection of potentially reversible delayed paraplegia may be achieved by MEP and SSEP monitoring with immediate assessment of function postoperatively. It is not our routine practice to perform MEP or SSEP monitoring. The adoption of the following provocative techniques for reduction of spinal cord ischemic-induced injury has resulted in a significant decrease in the incidence of paralysis following open repair that is dependent on the extent of repair: 15% for type I, 30% for type II, 7% for type III, and 4% for type IV. Additional predictors of delayed neurologic deficit for thoracoabdominal aneurysms include emergent operative status, prolonged aortic cross-clamp time, level of cross-clamp, hypogastric artery exclusion, aortic rupture, preoperative renal dysfunction, prior abdominal aortic aneurysm repair, acute dissection, and extent II involvement. These techniques and the avoidance of hemodynamic instability and significant blood volume loss have offset the influence of cross-clamp time on induced spinal cord injury.
 Fig. 59.4. Individual visceral vessel cannulation strategy for cardiopulmonary bypass and maintenance of visceral perfusion during thoracoabdominal aortic aneurysm reconstruction. |
Distal aortic perfusion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree