Congenital heart diseases are broadly defined as those cardiac anomalies that are present at birth. By their very nature, such defects have their origin in embryonic development. Most congenital cardiac lesions constitute gross structural abnormalities with a spectrum of associated hemodynamic derangements. It is not surprising that the various echocardiographic techniques are ideally suited to the study of patients with congenital heart disease. Perhaps nowhere in cardiology have these methods played a more vital role in diagnosis and management. Historically, the emergence of two-dimensional echocardiography must be viewed as a milestone in the diagnostic approach to congenital heart disease. The tomographic nature of the technique and the unlimited number of imaging planes permit the anatomy and relationships of the cardiac structures to be defined, even in the presence of complex congenital malformations. For the noninvasive assessment of cardiac structure and function, echocardiography plays a preeminent role as the most accurate and widely applied method.
The echocardiographic approach to patients with congenital heart lesions differs substantially from that used to evaluate other forms of cardiac disease. Imaging in children has both advantages and disadvantages compared with adults. The smaller patient size permits the use of higher frequency transducers, thereby enhancing image quality. The presence of less heavily calcified bone and the absence of hyperinflated lungs in most children increase the available acoustic windows and generally contribute to improved image quality. Unfortunately, the smaller patient size also creates practical problems for image acquisition. Children are more likely to be uncooperative and may have other malformations (such as a chest deformity) that complicate imaging.
Adults with congenital heart disease present an entirely different array of challenges to the echocardiographer. The decision to intervene in these patients frequently hinges on the adequacy of previous interventions and the presence and severity of pulmonary vascular disease. In patients who have undergone surgery, an accurate assessment may be difficult. When details of the clinical history are unavailable, the echocardiographer is often called on to determine which surgical procedures have been performed. The options for further intervention often depend on the echocardiographic results. As the patient with congenital heart disease ages, the superimposition of other medical conditions (such as hypertension or coronary disease) further complicates his or her evaluation and management. Both image acquisition and interpretation can be challenging and timeconsuming. The diversity and complexity of congenital cardiac malformations obviate even the most basic assumptions regarding chamber orientation and great vessel relationships. These problems are magnified in the patient who has undergone a surgical procedure previously. Therefore, the initial evaluation of the patient with suspected congenital heart disease mandates a thorough and systematic echocardiographic approach, often using additional views beyond those obtained during the standard examination.
This chapter focuses on the role of echocardiography in the adolescent and adult with congenital heart disease. Guidelines for the use of echocardiographic techniques in this growing patient population are provided in
Table 20.1. The chapter is not intended as an exhaustive description of all forms of congenital heart disease. Lesions that are seen more commonly in adult patients are emphasized, whereas those considered less relevant are covered only superficially. Finally, the evaluation of the postoperative patient is covered in some detail.
The Echocardiographic Examination: A Segmental Approach to Anatomy
The initial echocardiographic examination of the patient with suspected congenital heart disease requires a sequential and systematic approach to cardiac anatomy. Such a method is necessary to detect cardiac malpositions and to diagnose complex congenital heart disease. The first step in this sequential approach is to determine atrial situs and to assess the venous inflow patterns to the atria. Then, atrioventricular connections are defined and ventricular morphology and position are determined. Finally, ventriculoarterial relationships are evaluated. In most cases, this approach permits the identification of even the most complex forms of congenital heart disease (
Table 20.2).
Cardiac Situs
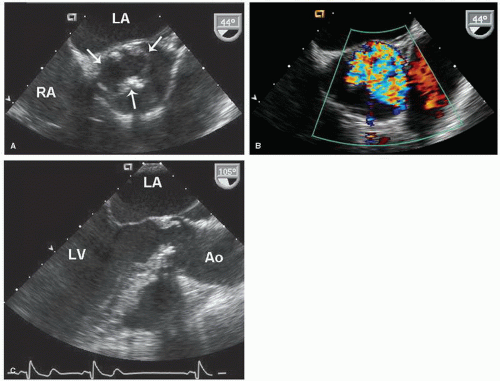
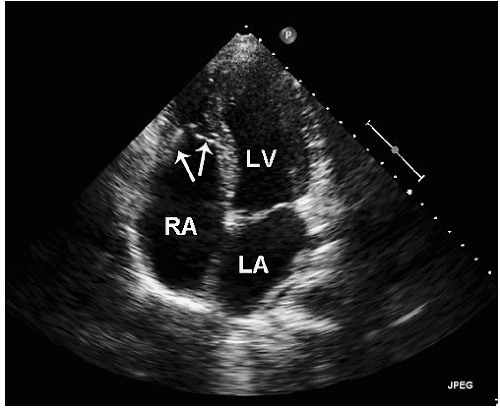
Determination of atrial situs is best accomplished by using the subcostal views. In atrial situs solitus, the normal situation, the morphologic right atrium is to the right and the morphologic left atrium is to the left. In situs inversus, the opposite occurs, creating a mirror image effect. Atrial and visceral situs are almost always concordant. Thus, a right-sided liver and left-sided stomach are usually associated with atrial situs solitus. In the rare cases when atrial and abdominal situs are discordant, however, the likelihood of complex congenital lesions is high. By using two-dimensional echocardiography, the location and morphology of the atria can be determined. The morphologic right atrium always contains the eustachian valve, and its appendage is shorter and broader than that of the left atrium. The left atrium lacks the eustachian valve and has a more rounded shape than the right atrium. The left atrial appendage is long and thin and has a narrower atrial junction than that of the right atrial appendage.
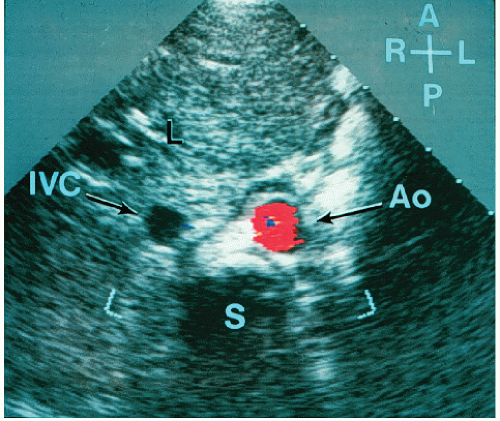
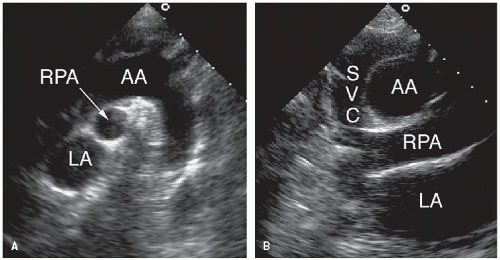
Although venous inflow does not define atrial morphology, the patterns of systemic and pulmonary venous return are helpful in determining situs. This spatial relationship is best evaluated using a transverse imaging plane through the upper abdomen. Normally, the abdominal aorta lies to the left and the inferior vena cava lies to the right of the spine. Compared with the vena cava, the aorta appears larger, more rounded, and more pulsatile. When in doubt, color flow imaging can be used to differentiate between the two vessels by demonstrating higher velocity and primarily systolic flow in the aorta (
Fig. 20.1). The opposite spatial relationship is characteristic of situs inversus. By tracing the course of the inferior vena cava and hepatic veins in the subcostal long-axis view, the right atrium generally can be identified in its usual position anterior and to the right of the left atrium (
Fig. 20.2).
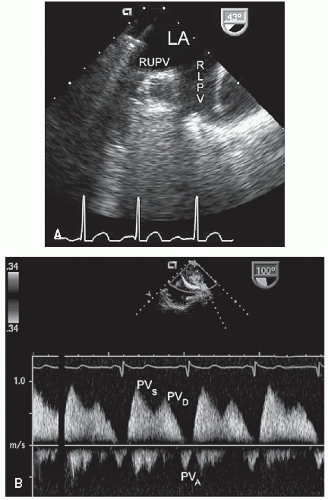
The pulmonary venous connections to the left atrium may be visualized using the apical and suprasternal window (
Fig. 20.3). Color Doppler imaging is particularly helpful in identifying the pulmonary veins as they enter the left atrium. In adults, it is usually impossible to record the insertion of all four pulmonary veins using transthoracic echocardiography. With transesophageal echocardiography, however, the pulmonary venous drainage pattern can be defined more precisely. Because of the possibility of anomalous pulmonary venous drainage, the relationship between the pulmonary veins and the left atrium is not constant, and their connections should not be used to define atrial morphology.
Ventricular Morphology
Once visceroatrial situs and venous connections are established, the orientation and morphology of the ventricles should be determined. During normal embryogenesis, the straight heart tube folds to the right (a D-loop) and then pivots to occupy a position within the left side of the chest. This positioning results in the right ventricle developing anteriorly and to the right of the left ventricle. The base-to-apex axis points leftward and most of the cardiac mass lies within the left side of the chest. If the initial fold in the heart tube is leftward, an L-loop develops, with the morphologic right ventricle to the left of the morphologic left ventricle. Thus, atrioventricular discordance occurs in the presence of situs solitus and an L-loop or situs inversus and a D-loop.
Ventricular morphology is readily assessed with twodimensional echocardiography. Features that are useful in distinguishing the right and left ventricles are listed in
Table 20.3. The presence of muscle bundles, particularly the moderator band, gives the right ventricle a trabeculated endocardial surface (
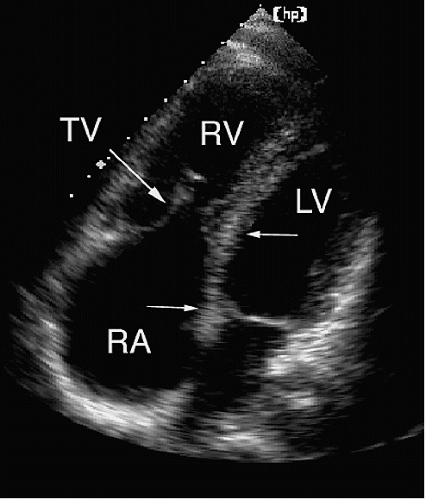
Fig. 20.4). In contrast, the left ventricle is characterized by a smooth endocardial surface. This distinction is apparent using echocardiography and serves as one of the more reliable characteristics when determining ventricular morphology. The structure and position of the atrioventricular valves are additional echocardiographic clues that are useful in distinguishing the right and left ventricles. If two ventricles are present, the
atrioventricular valves associate with the corresponding ventricle and identification of the mitral and tricuspid valves defines the respective chambers. The tricuspid valve is more apically displaced and has three leaflets (and three papillary muscles) and chordal insertions into the septum. The mitral valve has a more basal septal attachment and has two leaflets, which insert into two papillary muscles but not the septum. All these features can be assessed with echocardiography. The four-chamber view allows the echocardiographer to determine ventricular morphology and the relative positions of the atrioventricular valves. The short-axis views permit definition of the papillary muscles and chordal insertions. The relative positions of the atrioventricular valves and the presence or absence of chordal insertions into the septum are the most helpful echocardiographic features when attempting to determine ventricular identity.
Great Artery Connections
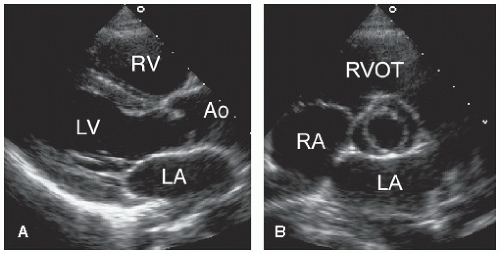
The final step in the segmental approach to cardiac anatomy involves identification of the great arteries and their respective connections. In the normal heart with concordant connections, the morphologic left ventricle gives rise to the aorta and the pulmonary artery serves as the outlet of the right ventricle. In the presence of normal ventricular orientation, this arrangement results in an anterior and leftward pulmonary artery and a posterior and rightward aorta with a left-sided aortic arch and descending aorta. The great arteries originate in orthogonal planes creating a “sausage and circle” appearance on short-axis imaging, which results from the rotation during development of the right ventricular outflow tract and pulmonary artery (the “sausage”) around the ascending aorta (the “circle”). Discordant ventriculoarterial connections, or transposition, occur when the great arteries arise from the opposite ventricle. Two forms of transposition exist. In D-transposition, ventricular relationship is normal, with the morphologic right ventricle located to the right of the morphologic left ventricle. In L-transposition, atrioventricular discordance is present (because of formation of an L-loop during embryogenesis) so that the morphologic right ventricle lies to the left of the morphologic left ventricle.
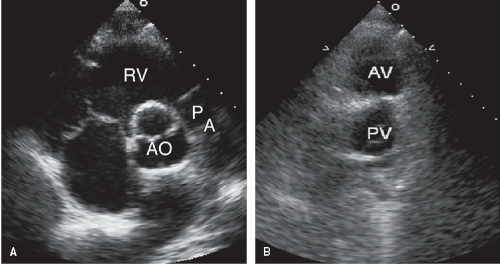
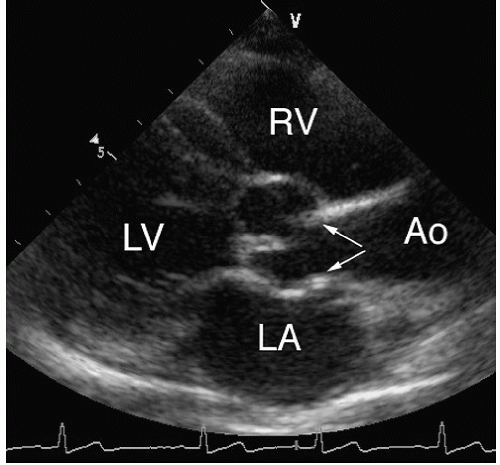
Two-dimensional echocardiography permits accurate identification of the great arteries and their origins and relationship. The short-axis view at the base of the heart is most helpful when assessing these features. In the normal heart, the pulmonary valve lies slightly anterior and to the left of the aortic valve (
Fig. 20.5). The pulmonary artery then courses posteriorly and bifurcates, with the right pulmonary artery passing immediately below the aortic arch. These findings are best appreciated in the parasternal long- and short-axis and subcostal views. The proximal aorta is optimally recorded from the parasternal window and the suprasternal notch (
Fig. 20.6). To identify the great arteries, the course of the vessel and the presence or absence of a bifurcation are the most reliable echocardiographic signs. The presence of a right aortic arch can also be detected by
assessing from the suprasternal short-axis view the course of the brachiocephalic vessels as they leave the arch.
Abnormalities of Right Ventricular Inflow
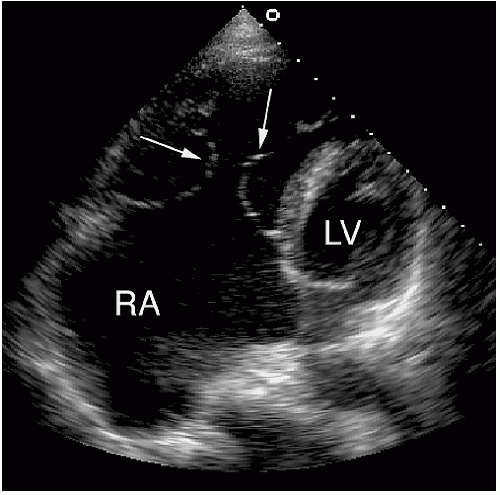
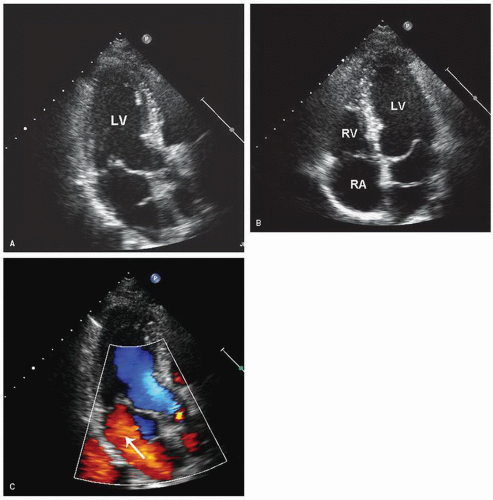
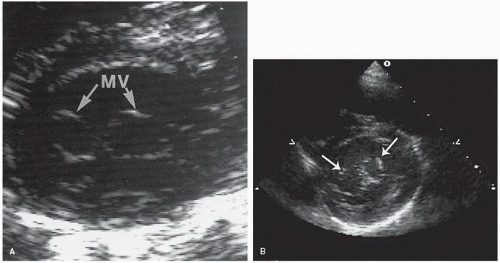
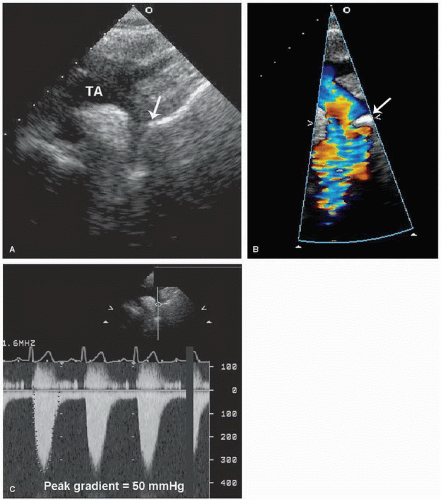
The right ventricular inflow tract and tricuspid valve are visualized using the apical and subcostal four-chamber views, the short-axis view at the base, and the medially angulated parasternal long-axis view. The most important congenital pathologic entities involving the tricuspid valve are Ebstein anomaly and tricuspid atresia (discussed subsequently). Ebstein anomaly consists of apical displacement of the septal and posterior (and sometimes the anterior) leaflets of the tricuspid valve into the right ventricle. Typically, the leaflets are elongated and redundant with abnormal chordal attachments. This results in “atrialization” of the basal portion of the right ventricle as the functional orifice is displaced apically relative to the anatomic annulus. Ebstein anomaly is a spectrum of abnormalities, depending on the extent of apical displacement of the valve, the distal attachments of the leaflets, the size and function of the remaining right ventricle, the degree of tricuspid regurgitation, and the presence of right ventricular outflow tract obstruction (usually from the redundant anterior tricuspid valve leaflet).
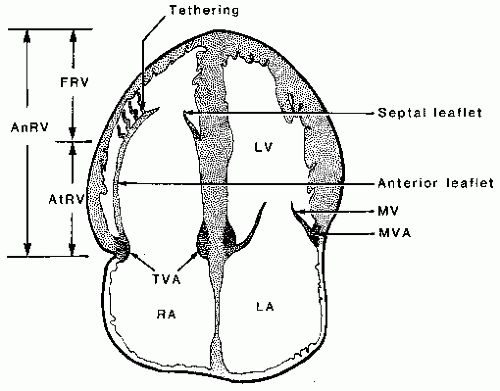
The best echocardiographic view for the evaluation of Ebstein anomaly is the four-chamber view. The characteristic features identified in this plane are shown schematically in
Figure 20.7. Of principal importance is the accurate recording of the level of insertion of the septal leaflet of the tricuspid valve relative to the annulus. Apical displacement of this insertion site is optimally assessed in this view and is the key to diagnosis (
Fig. 20.8). Because the tricuspid valve is normally positioned more apically than the mitral valve, abnormal apical displacement is relative, and some investigators have suggested measuring the distance between insertion sites of the two atrioventricular valves. When normalized for body surface area, a distance of greater than 8 mm/M
2 is indicative of Ebstein anomaly. Other
investigators have advocated a maximal displacement of more than 20 mm as the diagnostic criterion in adults.
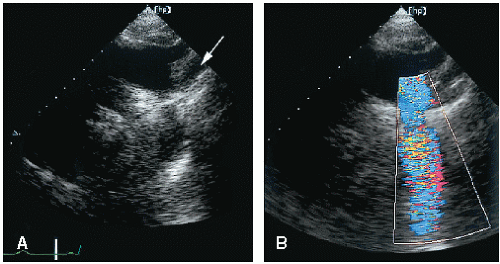
The four-chamber and medially angulated parasternal views may be used to assess the severity of Ebstein anomaly and to determine surgical options. The degree of atrialization of the ventricle, the extent of leaflet tethering, and the magnitude of deformity or dysplasia of the valve leaflets are important features with implications for surgical repair (
Fig. 20.9). The extent of chordal attachments between the anterior leaflet and the anterior free wall should be assessed in multiple views. If tethering is significant, valve replacement rather than repair may be required. The greater the degree of atrialization is, the worse the prognosis.
Figure 20.10 is an example of an extreme form of Ebstein anomaly, with displacement of the tricuspid leaflets well into the right ventricular apex and marked tethering of the valve tissue. If the area of the functional right ventricle is less than one third of the total right ventricular area, overall prognosis is poor. Because of the complexity of right ventricular geometry, an accurate measure of the size of the functional right ventricle is difficult, and all available views should be used. Doppler echocardiography should be used to detect tricuspid regurgitation, which is commonly seen in patients with Ebstein anomaly (
Fig. 20.11). A redundant anterior tricuspid valve leaflet may cause functional right ventricular outflow tract obstruction, which can also be detected with Doppler imaging. In severe cases, pulmonary atresia may be present, although it is rarely seen in adults.
Ebstein anomaly may be associated with a variety of other abnormalities that can be detected with echocardiography, namely, atrial septal defect, mitral valve prolapse, and left ventricular dysfunction. The etiology of the left ventricular dysfunction is not known, but its presence is associated with a poor prognosis. Surgical options in patients with Ebstein anomaly include tricuspid valve repair or replacement. After surgical repair, echocardiography plays a role in assessing the success of the procedure and the function of the tricuspid valve.
Abnormalities of Right Ventricular Outflow
Right Ventricle
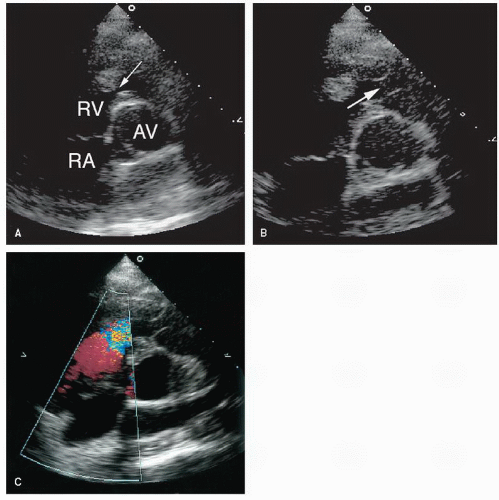
Narrowing of the right ventricular outflow tract can occur on several levels, and obstruction may be present at multiple sites within an individual patient. Subvalvular pulmonary stenosis usually involves the infundibulum and is less common than valvular stenosis. Infundibular pulmonary stenosis may be the result of discrete fibromuscular narrowing or hypertrophied subvalvular muscle bundles (also called doublechambered right ventricle) (
Fig. 20.21). In many cases, a ventricular septal defect is also present. Right ventricular outflow tract narrowing is occasionally secondary to stenosis at a more distal level. For example, valvular pulmonary stenosis may lead to right ventricular hypertrophy, the development of subvalvular muscle bundles, and subsequent outflow tract narrowing.
Two-dimensional echocardiography is well suited to the evaluation of the right ventricular outflow tract. The parasternal short-axis and the subcostal four-chamber views are ideal for assessing the complex geometry of this region and for determining the level and severity of stenosis. The use of Doppler imaging to measure the pressure gradient may be challenging, however. Orienting the ultrasound beam parallel to the outflow tract jet requires considerable effort and the use of all available windows. Furthermore, localization of the site of stenosis may be difficult if narrowing occurs at more than one level. Typically, subvalvular stenosis is a dynamic form of obstruction with maximal velocity occurring in late systole, a pattern that is analogous to the outflow jet seen in hypertrophic cardiomyopathy. The magnitude of reduction in pulmonary artery flow can affect development of the pulmonary arteries, which can be an important factor in surgical planning. Therefore, an evaluation of children with any form of right ventricular outflow tract obstruction should include an assessment of the pulmonary arteries. This includes patients with tetralogy of Fallot, in whom the type and timing of surgical repair are determined in part by the size of the pulmonary arteries.
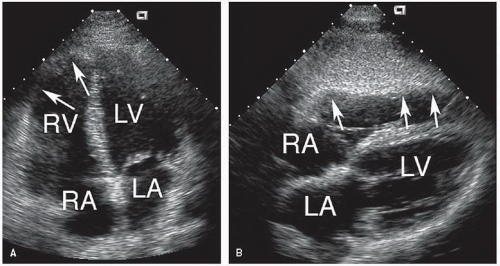
A rare congenital abnormality of the right ventricle is arrhythmogenic right ventricular cardiomyopathy (
Fig. 20.22). This condition is characterized by dysplasia of the right ventricular myocardium, the extent of which varies considerably. Functionally, the dysplastic myocardium results in a form of right ventricular cardiomyopathy with decreased contractility and a propensity for ventricular arrhythmias. A spectrum of echocardiographic findings exists, depending on the extent of involvement. Thinning and hypokinesis of the free wall are characteristic. The systolic dysfunction may appear regional or, in cases of extensive dysplasia, global. Associated valvular pathology is not a feature of this condition.
Pulmonary Valve
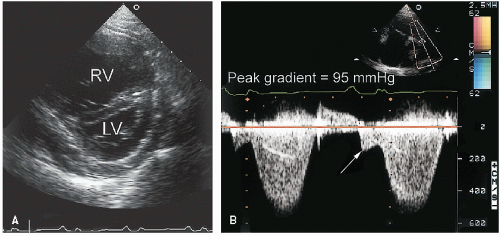
Stenosis of the pulmonary valve is a fairly common congenital lesion that may occur in isolation or in association with other cardiac defects. The most frequently encountered form is characterized by fusion of the cusps and incompletely formed raphae, resulting in a domelike structure with a narrowed orifice. Typically, the valve annulus is normal in size. With severe stenosis, right ventricular hypertrophy may lead to variable degrees of subvalvular narrowing.
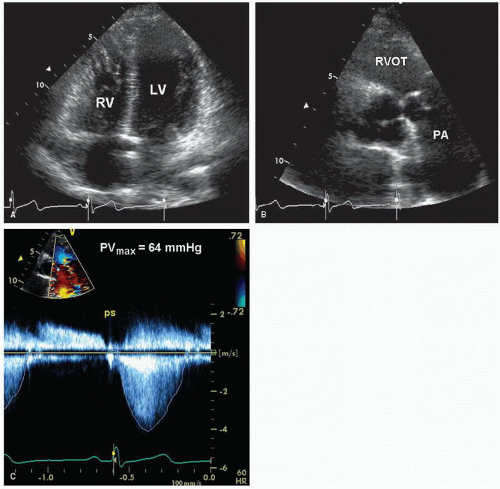
In adults, the morphology of the stenotic pulmonary valve is best visualized in the parasternal short-axis plane through the base of the heart. With two-dimensional echocardiography, the cusps appear thickened, have decreased excursion, and dome in systole (
Fig. 20.23). Poststenotic pulmonary artery dilation is frequently evident, but its presence does not correlate with severity. In most cases, right ventricular size and function are normal, and trabeculation of the right ventricular walls is increased (see
Fig. 20.23A). Calcification of the valve is characteristic in adults, but not children, with this disorder. Less common, dysplasia of the pulmonary valve will cause valvular stenosis at birth due to myxomatous thickening of the leaflets (
Fig. 20.24). When pulmonary stenosis is severe, evidence of right ventricular pressure overload will be present. The degree of septal flattening and right ventricular enlargement correlate roughly with the severity of stenosis.
Figure 20.25 is an example of extreme right ventricular pressure overload secondary to severe valvular pulmonary stenosis.
Although two-dimensional echocardiography is essential for the morphologic diagnosis of pulmonary stenosis, the technique is limited for assessing the severity of obstruction. Neither the degree of cusp thickening nor the presence of right ventricular hypertrophy provides a quantitative measure of severity. Doppler imaging is the technique of choice to measure the severity of pulmonary stenosis. Using the modified Bernoulli equation, the peak instantaneous pressure gradient can be calculated (
Figs. 20.23,
20.24 and
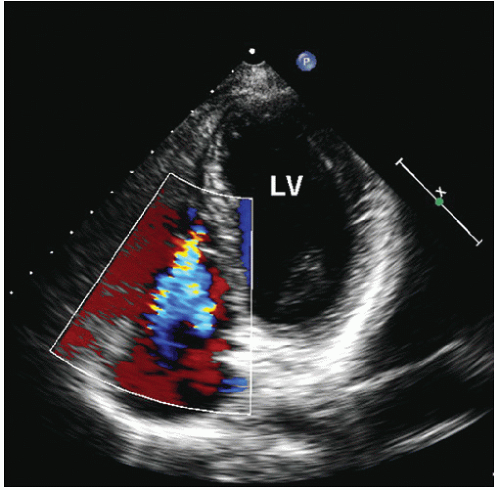
20.25). Several clinical studies have demonstrated an excellent correlation between Doppler imaging and catheterization-derived pressure gradients in patients with pulmonary stenosis. In most patients, optimal alignment of the Doppler beam with the stenotic jet uses the parasternal short-axis view. In some individuals, use of a lower interspace is necessary to better align with a superiorly directed jet. In patients with pulmonary artery dilation, anterior displacement of the valve precludes proper beam alignment from the parasternal window. In this situation, the subcostal or suprasternal approach is usually adequate. In children, particularly, the subcostal approach provides optimal beam alignment and permits detection of the maximal jet velocity.
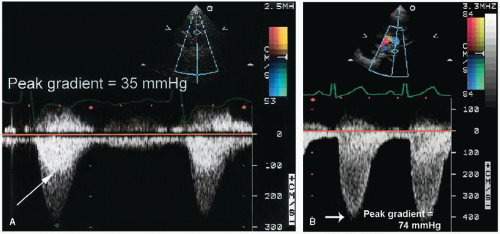
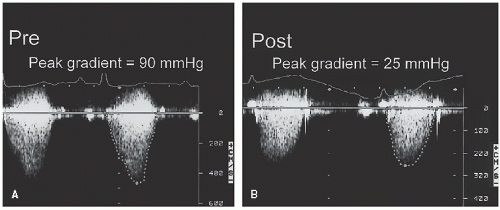
In children with pulmonary stenosis, surgical valvotomy or balloon valvuloplasty is often performed to relieve the obstruction. After such interventions, Doppler echocardiography may be used for serial evaluation and to detect residual stenosis (
Fig. 20.26). The magnitude of associated pulmonary insufficiency and abnormalities of right ventricular diastolic filling can also be assessed. In patients with combined valvular and infundibular stenosis, the presence of serial obstructions may result in overestimation by continuous wave Doppler imaging of the catheterization-derived pressure gradient.
Pulmonary Artery
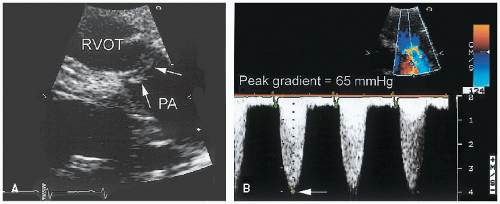
Pulmonary artery stenosis (also referred to as peripheral or supravalvular pulmonary stenosis) can occur at any level and often involves multiple sites. Several morphologic forms exist, including discrete membranelike lesions, long tubular stenoses, and tubular hypoplasia. These anomalies frequently are associated with other congenital cardiac and extracardiac lesions (e.g., Williams syndrome). The ability to detect pulmonary artery stenoses with echocardiography depends on the location
of the lesions. Proximal lesions can be visualized from the parasternal short-axis window.
Figure 20.27 is an example of peripheral pulmonary stenosis involving the right branch. In most such cases, the diagnosis is apparent from twodimensional echocardiographic imaging. Color Doppler imaging should be used to demonstrate turbulence and acceleration of flow within the stenotic segment. The echocardiographer must bear in mind, however, that a more common cause of turbulent flow within the main pulmonary artery is patent ductus arteriosus. More peripheral stenoses may be impossible to visualize, especially in older patients. In children, the subcostal four-chamber and the suprasternal views may permit detection of distal lesions. The diagnosis should be considered in a patient with unexplained right ventricular hypertrophy, particularly in the presence of a pulsatile proximal pulmonary artery.
Abnormalities of Left Ventricular Outflow
Congenital abnormalities of left ventricular outflow usually involve obstruction of flow, and several important forms exist.
These lesions may be categorized as subvalvular, valvular, or supravalvular (which includes coarctation of the aorta) (
Table 20.5). The subvalvular forms are heterogeneous and include hypertrophic cardiomyopathy, which is discussed in
Chapter 19. The most important forms are the valvular lesions, which are common causes of stenosis in children (the unicuspid or congenitally stenotic aortic valve) and in adults (the bicuspid valve). The form of supravalvular obstruction encountered most frequently in the adult patient is coarctation of the aorta. This section includes a discussion of the lesions that occur at each of these different levels in order, but the focus is on those anomalies that are most common in adults.
Subvalvular Obstruction
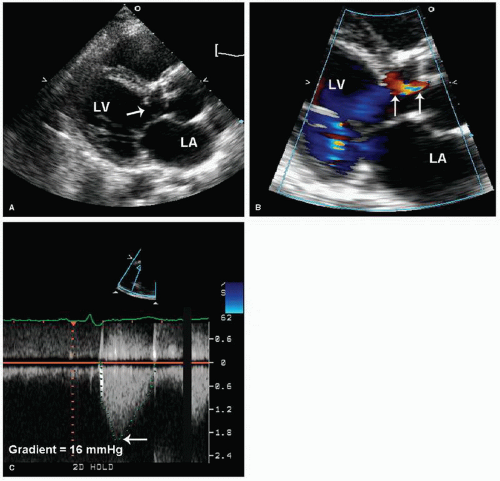
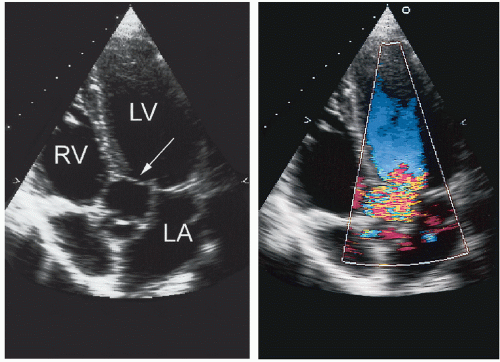
Two types of subvalvular aortic stenosis are discussed here: the discrete form and the fibromuscular type of subaortic obstruction. Together, these lesions account for less than 20% of
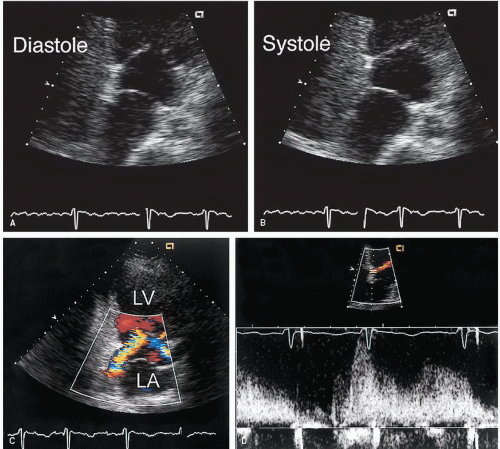
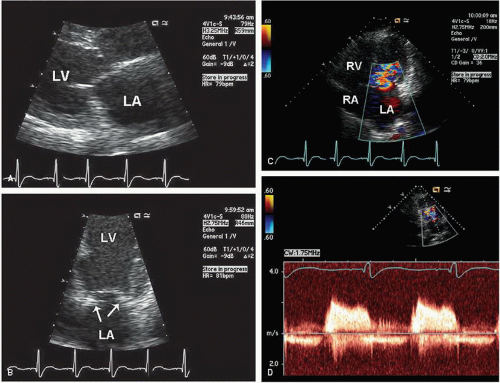
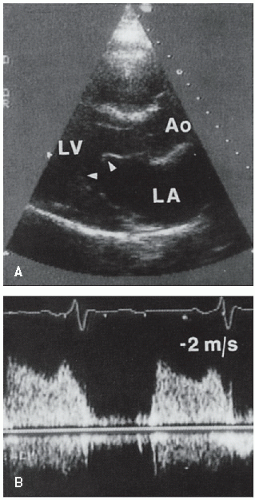
all cases of left ventricular outflow obstruction in children and both are uncommon in adult patients. Discrete subaortic stenosis results from a thin, fibrous membrane or ridge that forms a crescentic barrier within the outflow tract just below the aortic valve. The membrane usually extends from the anterior septum to the anterior mitral leaflet. The degree of obstruction to flow is variable, and aortic regurgitation develops in approximately 50% of patients. With two-dimensional echocardiography, these membranes are seen as a discrete linear echo in the left ventricular outflow tract perpendicular to the interventricular septum. Because the membranes are parallel to the beam, recording these structures from the parasternal long-axis window may require the use of multiple transducer positions (
Fig. 20.28). In many cases, the membranes are detected more easily from the apical views (where the ultrasound beam is oriented perpendicular to the structure) (
Fig. 20.29). Transesophageal echocardiography has also been used in the assessment of patients with subvalvular obstruction. Doppler imaging plays an essential role in the evaluation of these patients. After the location and orientation of the jet are visualized with color flow imaging, continuous wave Doppler imaging can be used to estimate the peak pressure gradient across the membrane (
Fig. 20.30). In the absence of aortic valve stenosis, this value correlates well with the catheterization-derived measure of obstruction. In the presence of multiple serial stenoses, however, Doppler imaging may overestimate the catheterizationmeasured gradient. The presence and severity of aortic regurgitation can also be assessed with Doppler techniques (
Fig. 20.28).
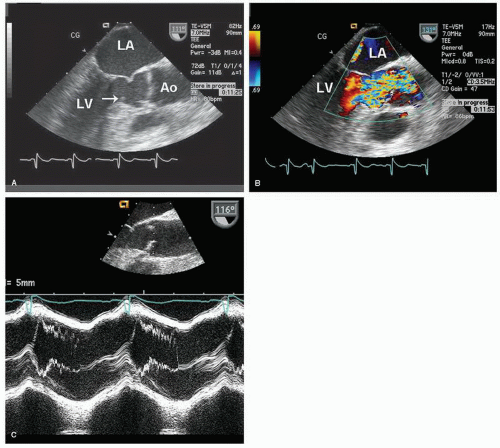
Figure 31 is an example of a subaortic membrane evaluated with transesophageal imaging. Note how the attachment of the membrane to the anterior mitral leaflet deforms the valve, especially during systole. M-mode echocardiography can also be helpful in assessing subvalvular obstruction (
Fig. 31C). Midsystolic partial closure with reopening of the leaflets in late systole is indicative of a subvalvular pressure gradient.
Membranous subaortic stenosis is distinguished from a subaortic fibromuscular ridge or tunnel with two-dimensional echocardiography. Tunnel-type subaortic obstruction, rarely seen in adults, is characterized by diffuse thickening and narrowing of the left ventricular outflow tract with associated concentric left ventricular hypertrophy. A fibromuscular ridge may also obstruct the outflow tract (
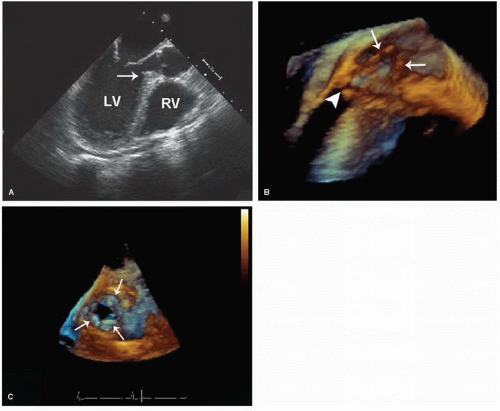
Fig. 20.30). This entity is similar to discrete membranous subaortic stenosis, but the obstruction is thicker and less discrete and appears more muscular.
Figure 20.32 is an example of a fibromuscular ridge evaluated with transesophageal three-dimensional imaging. The improved spatial orientation provided by the three-dimensional technique allows a more complete characterization of the outflow tract and type of obstruction.
These different forms of subaortic obstruction probably exist as a continuum, with a thin discrete membrane at one extreme and a diffuse tunnel at the other. Differentiating among individual cases may, therefore, be difficult and somewhat arbitrary. All these forms of subaortic obstruction are frequently associated with ventricular septal defects. Occasionally, other congenital cardiac anomalies are associated with subvalvular left ventricular outflow tract obstruction, including accessory mitral valve chordae, anomalous papillary muscle insertion, and abnormal insertion of the anterior mitral leaflet.
Valvular Aortic Stenosis
Aortic stenosis may be present at birth (a congenitally stenotic aortic valve) or may develop over time in a congenitally abnormal, but not stenotic, valve. In the former, the valve may be acommissural (resembling a volcano and more typical of pulmonary stenosis) or unicuspid unicommissural (with a slitlike orifice, resembling an exclamation point,
Fig. 20.33). A bicuspid or tricuspid valve can also be stenotic at birth because of commissural fusion or dysplasia. Most often, such valves will be functionally normal at birth but gradually become stenotic over time because of progressive fibrosis and calcification. In other cases, degeneration of the valve leads to predominant aortic regurgitation. Quadricuspid valves are rare and have a similar natural history.
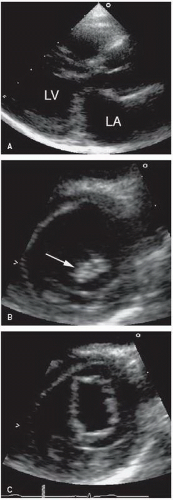
Bicuspid aortic valve is estimated to occur in 1% to 2% of the general population, making it the single most common congenital cardiac anomaly. As just noted, these valves often are functionally normal at birth (
Fig. 20.34). Two-dimensional echocardiography plays a major role in detection of this entity. Direct visualization of the aortic cusps is possible from the parasternal short-axis view through the base of the heart. During diastole, the cusps of a normal tricuspid valve are closed within the plane of the scan and the commissures form a “Y” (sometimes referred to as an inverted Mercedes-Benz sign). A true bicuspid valve has two cusps of nearly equal size, two associated sinuses, and a single linear commissure. A raphe may be present and, if present, creates the illusion of three separate cusps. By observing valve opening in systole, however, the number of distinct cusps is apparent. Fusion of two of the cusps may create the appearance of a bicuspid valve, but the presence of three
distinct sinuses will establish this difference. Confirming the presence of a bicuspid aortic valve with echocardiography requires high-resolution images from the short-axis view for adequate visualization of valve morphology. A unicuspid valve has a single slitlike commissure, and the opening is eccentric and restricted. The stenotic tricuspid valve has three cusps with variable degrees of commissural fusion. Thus, an accurate assessment of functional anatomy requires an analysis of the number of apparent cusps, the degree of cusp separation, and a recording of their mobility and excursion during systole.
Whereas the short-axis view is useful for determining the number of commissures and the degree, if any, of commissural fusion, movement of the cusps out of the imaging plane during systole precludes accurate determination of the presence and severity of stenosis. In fact, normal systolic excursion of the bodies of the cusps recorded from the short-axis view may lead to underestimation of the severity of congenital aortic stenosis. Thus, the short-axis view is useful when evaluating aortic valve anatomy but should never be used to exclude the possibility of congenital aortic stenosis. The long-axis views have several advantages for this purpose. The thickness and excursion of the cusps can be assessed. Normally, they appear as thin, delicate structures that appear to open completely in systole and are aligned parallel to and against the aortic walls. With congenital aortic stenosis, the cusps are thickened and appear to dome during systole, the result of restricted motion of the tips relative to the more mobile bodies of the cusps (
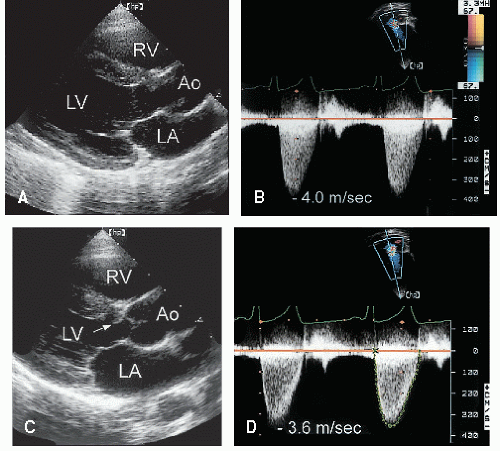
Fig. 20.35). A qualitative estimate of severity is possible, based on the thickness and immobility of the cusps, the extent of leaflet tip separation in systole, the degree of left ventricular hypertrophy, and the presence of poststenotic aortic root dilation.
Doppler imaging should be used to complete the noninvasive assessment of aortic stenosis and to provide a quantitative evaluation of severity. The apical, right parasternal, and suprasternal windows should be used to ensure that the maximal velocity is obtained. Then, through the use of the modified Bernoulli equation, the peak pressure gradient can be calculated. Both peak instantaneous and mean pressure gradients can be derived, and in children, the mean gradient is often used for clinical decision making. The values obtained with this approach correlate well with catheterization-derived gradients. Inherent differences exist between the two methods, and discrepancies should not necessarily be viewed as an error on the part of one or the other technique. In children especially, anxiety and increased activity during the examination will lead to an increase in flow velocity (both proximal and distal to the valve) and will thereby increase the measured pressure gradient. To calculate aortic valve area, the continuity equation can be used. It should be emphasized that the application of Doppler imaging to quantify aortic stenosis is similar in children and adults. The basic
principles underlying these applications are covered in detail in
Chapters 9 and
11.
Supravalvular Aortic Stenosis
The least common site for congenital aortic stenosis is in the supravalvular area. Three morphologic types of supravalvular aortic stenosis have been described: (1) fibromuscular thickening producing an hourglass-shaped narrowing above the sinuses (the most common form), (2) a discrete fibrous membrane in a normal-sized aorta, usually located near the sinotubular junction, and (3) diffuse hypoplasia of the ascending aorta, often involving the origins of the brachiocephalic arteries. Because of the presence of stenosis above the aortic valve and coronary ostia, two additional features often accompany these anomalies: (1) dilation of the coronary arteries, sometimes with ostial obstruction and (2) thickening and fibrosis of the aortic cusps, usually with an element of aortic regurgitation. Williams syndrome includes supravalvular aortic stenosis, elfin facies, mental retardation, and, occasionally, peripheral pulmonary stenosis. Isolated supravalvular aortic stenosis with or without
peripheral pulmonary stenosis may be inherited as an autosomal dominant trait.
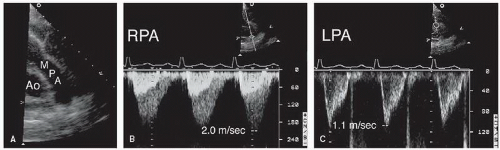
The parasternal long-axis view or a high right parasternal view is most helpful for diagnosing supravalvular aortic stenosis. In the normal aorta, the vessel diameter is greatest at the level of the sinuses. At the sinotubular junction, the diameter decreases slightly and approximates the size of the aortic annulus. With supravalvular aortic stenosis, an hourglass deformity occurs that is characterized by a segment of gradual tapering and then widening of the lumen (
Fig. 20.36). The aortic walls usually appear thickened and echogenic. Aortic cusp fibrosis is often present, but poststenotic dilation of the ascending aorta is not a feature of this anomaly. A hypoplastic aorta is characterized by more diffuse and extensive narrowing with variable involvement of the branch vessels.
Assessing the severity of supravalvular aortic stenosis relies on two-dimensional echocardiography for accurate visualization of the magnitude and linear extent of the narrowing. Careful assessment of the aortic valve and the coronary arteries is an essential part of the evaluation of these patients. Proximal coronary artery dilation or ostial stenosis may be detected from the parasternal short-axis view at the base of the heart. Doppler imaging can be used to estimate the peak pressure drop across the site of aortic narrowing. In the presence of a discrete, isolated stenosis, the pressure gradient derived from Doppler imaging is an accurate reflector of severity. As noted previously, however, if the stenoses are multiple or tubular, the correlation between Doppler imaging and catheterization-derived gradients may be poor.
Coarctation of the Aorta
This relatively common condition is the result of localized narrowing of the descending aorta near the origin of the ductus arteriosus. The lesion consists of a ridgelike indentation of the posterolateral wall of the aorta resulting from thickening and infolding of the aortic media. It is typically located just distal to the origin of the left subclavian artery and the specific location may be “preductal” or “postductal” depending on the position of the ridge of tissue relative to the ductus (or ligamentum) arteriosus. It is often associated with other forms of congenital heart disease, especially bicuspid aortic valve and mitral valve malformations.
Echocardiographic detection of coarctation requires both an index of suspicion and careful recording of the descending aorta from the suprasternal window. In children, the evaluation of this portion of the aorta is relatively straightforward. In adults, however, the assessment can be technically demanding and both false-negative and false-positive results occur. The goal is to record the arch and descending aorta in the long axis from the suprasternal notch. False-negative results usually result from an inability to image the most distal portion of the arch (where the narrowing occurs). False-positive findings are the result of a tangential imaging plane through the vessel, creating the illusion of narrowing. The origins of the carotid and subclavian arteries serve as landmarks when localizing the juxtaductal area. The location of the left subclavian artery relative to the coarctation is an important factor in surgical management. If an area of stenosis is suspected, care should be taken to ensure proper beam alignment. If the aortic lumen can be seen beyond the narrowing, the likelihood of a false-positive result is reduced (
Fig. 20.37). Dilation and exaggerated pulsation of the proximal aortic arch are further evidence of significant coarctation.
An example of coarctation of the aorta in an adult patient is shown in
Figure 20.38. Note the location of a shelflike constriction just beyond the origin of the left subclavian artery. Dilation of the ascending aorta is also apparent. When two-dimensional echocardiographic imaging is diagnostic of (or suspicious for) coarctation, Doppler imaging should be performed to aid in the diagnosis and to provide an estimation of the pressure gradient. As a first step, color Doppler imaging can be used to detect acceleration and turbulence within the region of narrowing. The absence of Doppler evidence of acceleration and turbulence of flow should alert the examiner to the possibility of a false-positive two-dimensional echocardiographic result. Color Doppler imaging also permits more accurate alignment of the continuous wave Doppler beam.
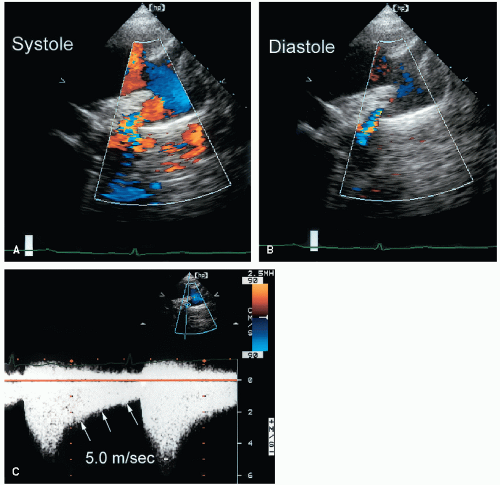
Figure 20.39 includes two examples of Doppler recordings of flow across an aortic coarctation. To estimate the peak pressure gradient, the Bernoulli equation can be used. When this equation is applied to aortic coarctation, however, it may be inappropriate to ignore the proximal aortic flow velocity. As a general rule, if this proximal velocity is less than 1.5 m/sec, it can be ignored and the simplified equation can be used. If it is greater than 1.5 m/sec, the expanded Bernoulli equation is necessary. In this way, a more accurate pressure gradient is obtained. The persistence of a high-velocity flow signal into diastole is another useful clue to the severity of the stenosis. A pressure gradient throughout the cardiac cycle indicates a more severe form of obstruction compared with a pressure gradient that is confined to systole (
Fig. 20.40). In this example, color Doppler imaging reveals persistence of turbulent antegrade flow across the coarct. Then, the presence of a diastolic gradient is confirmed with continuous wave Doppler imaging. Because coarctation gradients are flow dependent, low-level exercise, usually in the form of leg lifts, can be performed to assess the response to stress. In many cases, exercise will not cause a significant increase in the peak gradient but will result in the development or increase in the diastolic gradient. In borderline cases, this response can be helpful in clinical decision making.
Although Doppler imaging is sensitive for the detection of coarctation, false-negative results can occur in the presence of a patent ductus arteriosus. Left-to-right runoff of blood flow through the ductus reduces the jet velocity through the coarctation and leads to an underestimation of the pressure gradient. This can also occur in the presence of welldeveloped collaterals. In such cases, the Doppler gradient will be an underestimation of the actual severity of obstruction.
False-positive results are even less common. Occasionally, a mild increase (1.5-2 m/sec) in descending aortic flow velocity will be misinterpreted as evidence of coarctation. In the absence of turbulence or echocardiographic evidence of vessel narrowing, this should generally be attributed to normal acceleration around the arch. Long-term follow-up after repair of aortic coarctation relies heavily on echocardiographic methods for the detection of restenosis. Estimation of the restenosis gradient by Doppler imaging is possible and correlates well with catheterization-derived values (
Fig. 20.41).
Aortic atresia and interrupted aortic arch are severe and uncommon forms of left ventricular outflow obstruction. They may be diagnosed in utero or shortly after birth by using echocardiographic techniques. Interruption of the aortic arch may be thought of as an extreme form of coarctation. The length of the “missing” segment varies, as do the relative insertion sites of the arch vessels. With echocardiography, the diagnosis rests on visualization of the aortic arch as it abruptly terminates, and
it is usually best seen from the suprasternal window. A patent ductus arteriosus (usually large) will also be present. When aortic arch interruption is suspected, a careful search for a right aortic arch should be undertaken to avoid confusion between these two entities.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access