Myocardial ischemic origin is a significant independent predictor of mortality in patients with heart failure (HF). The implications of angina pectoris (AP) in HF are less well characterized. The aim of this study was to compare the clinical characteristics and outcomes of patients with and without AP in a cohort of patients with reduced ejection fractions and ischemic cardiomyopathy (iCM). Patients who underwent coronary angiography at Duke University Medical Center from January 2000 to September 2009 with ejection fractions <40% and diagnoses of iCM with AP in the previous 6 weeks were compared to similar patients without AP. Time to event was examined using Kaplan-Meier methods for 5 end points: death; death or nonfatal myocardial infarction (MI); death, MI, or revascularization; death or hospitalization; and cardiovascular (CV) death or CV hospitalization. Of 2,376 patients with iCM, 1,412 (59%) had AP. They had more co-morbidities and more previous revascularization than patients without AP. After multivariate adjustment, those with and without AP had similar risks for death (p = 0.32), death or MI (p = 0.15), and death or hospitalization (p = 0.37) (5-year event rates 41% vs 41%, 46% vs 47%, and 87% vs 85%, respectively), but those with AP had lower rates of death, MI, or revascularization (p = 0.01) and higher rates of CV death or CV hospitalization (p = 0.03) (5-year event rates 85% vs 87% and 77% vs 73%, respectively). In conclusion, AP is common in patients with iCM despite medical therapy and previous revascularization and is associated with increased CV death or CV rehospitalization.
Heart failure (HF) is a major public health problem worldwide with high morbidity, mortality, and cost. Despite recent therapeutic advances, the overall prognosis of patients with HF remains poor, with 5-year mortality approaching 50%. Because of management and prognostic implications, patients with HF are commonly divided into ischemic versus nonischemic origin. However, conflicting data exist on this association. Angina pectoris (AP) is the symptomatic condition related to myocardial ischemia and has been regarded as having a variable prognosis. In the general population, there is increasing recognition that those with chronic stable AP may have a fairly good prognosis and that invasive procedures may reduce symptoms but not improve prognosis compared to aggressive medical management. Conversely, a large cohort study of 8,908 outpatients with coronary artery disease found that AP consistently predicted mortality. The implications of AP in HF are not well defined, because patients with HF have generally been excluded from AP studies. Nonetheless, AP is common in patients with HF. We compared the clinical characteristics and outcomes of patients with and without AP in a cohort of patients with HF with ischemic cardiomyopathy (iCM).
Methods
Patient data were obtained from the Duke Databank for Cardiovascular Disease, an ongoing databank of all patients who undergo diagnostic cardiac catheterization at Duke University Medical Center. Patients were included in the study population if they underwent coronary angiography from January 2000 to September 2009 and had left ventricular ejection fractions <40% and histories of ≥50% stenosis in ≥1 epicardial coronary vessel (only those patients with histories of significant coronary artery disease receive Duke Databank for Cardiovascular Disease follow-up). Coronary stenoses were graded by visual consensus of ≥2 experienced observers. We used the standardized research definition of iCM previously shown to be the most prognostically powerful: (1) a history of myocardial infarction (MI) or revascularization (coronary artery bypass grafting or percutaneous coronary intervention), (2) ≥75% stenosis of the left main or proximal left anterior descending coronary artery, or (3) ≥75% stenosis of ≥2 epicardial vessels. Patients were excluded from analysis if they had primary valvular heart disease (defined as severe aortic or mitral insufficiency or severe stenosis of any heart valve), congenital heart disease, acquired immunodeficiency syndrome, or metastatic cancer. Data from the index catheterization were prospectively collected as part of routine patient care. Baseline clinical variables for each patient were stored in the Duke Databank for Cardiovascular Disease using methods previously described. Follow-up was obtained through self-administered questionnaires, with telephone follow-up of nonresponders. Patients not contacted through this mechanism had vital status determined through a search of the National Death Index.
For this analysis, revascularization was defined as treatment with percutaneous coronary intervention or coronary artery bypass grafting. Mode of death and rehospitalization were determined using methods previously described. In brief, cardiovascular (CV) death included death occurring suddenly, after resuscitation, during cardiac catheterization, during or after cardiac surgery, or due to MI, HF, vascular causes, or other cardiac death not otherwise specified. Noncardiac deaths were those classified as noncardiac medical (e.g., cancer, liver disease, pulmonary disease), trauma, and noncardiac procedural. Hospitalizations were classified as CV or not on the basis of diagnosis-related groups for Duke hospital admissions or indications noted on the follow-up surveys for non-Duke hospitalizations (e.g., International Classification of Disease, Ninth Revision, codes and/or procedure codes). CV hospitalization included hospitalization for revascularization.
AP classification was based on physician-obtained patient history just before cardiac catheterization and was defined as chest pain within the previous 6 weeks. Because many groups (e.g., women, elderly) present with atypical angina, we did not want to bias our results by using a classic angina definition alone. Given the prognostic value of angina characteristics, the severity, frequency, and pattern of occurrence were recorded at baseline.
Baseline characteristics are described with medians and interquartile ranges for continuous variables and percentages for discrete variables. Unadjusted time-to-event results were assessed using Kaplan-Meier methods, and comparisons were made using the log-rank test for 5 end points: death; death or nonfatal MI; death, MI, or revascularization; death or hospitalization; and CV death or CV hospitalization. A p value <0.05 was used to indicate statistical significance for all comparisons. Multivariate Cox proportional-hazards regression analysis was used to adjust for baseline differences between groups. Statistical analyses were performed independently by the Duke Clinical Research Institute (Durham, North Carolina) using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
A total of 2,376 patients met the criteria for the study ( Figure 1 ) , with a median follow-up period of 4.5 years (interquartile range 1.85 to 7.18); there was no significant difference in follow-up between the AP and non-AP cohorts.
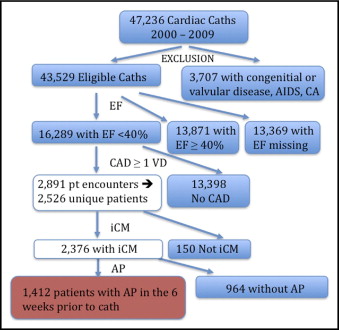
Baseline characteristics for the AP and non-AP groups are listed in Table 1 . There were 1,412 patients (59%) with AP and 964 (41%) without AP. In the AP cohort, 73.0% had typical angina and 26.6% had atypical angina in the previous 6 weeks. AP was described as stable, progressing, and unstable in 12.6%, 37.2%, and 48.8% of patients in the preceding 6 weeks, respectively. Using a modification of the Canadian Cardiovascular Society angina grade, the percentages of patients with AP with Canadian Cardiovascular Society class I (no symptoms with ordinary activity), class II (symptoms with moderate exertion), class III (symptoms with ordinary exertion), class IV (symptoms with any exertion or at rest), and symptoms unrelated to exertion were 0.4%, 10.1%, 11.9%, 40.7%, and 36.9%, respectively. The median frequency per week of chest pain episodes was 3 (interquartile range 2 to 7). As expected, a number of baseline characteristics differed significantly between the cohorts ( Table 1 ). The 2 groups had high baseline use of β blockers, aspirin, and statins but low use of calcium channel blockers, nitrates, and hydralazine.
| Variable | AP (Past 6 Weeks) | p Value | |
|---|---|---|---|
| No (n = 964) | Yes (n = 1,412) | ||
| Age (years) | 64 (56–73) | 64 (55–73) | 0.48 |
| Men | 74.7% | 74.4% | 0.89 |
| Caucasian | 69.6% | 72.6% | 0.11 |
| Hypertension | 66.6% | 73.3% | <0.001 ⁎ |
| Diabetes mellitus | 33.9% | 36.5% | 0.20 |
| Previous MI | 71.5% | 71.8% | 0.86 |
| Dyslipidemia † | 52.8% | 66.6% | <0.001 ⁎ |
| 3-vessel coronary disease | 54.7% | 59.6% | 0.02 ⁎ |
| Ejection fraction (%) | 31 (25–36) | 33 (27–37) | 0.001 ⁎ |
| New York Heart Association class III or IV | 27.8% | 21.0% | <0.001 ⁎ |
| Cerebrovascular disease | 11.8% | 13.5% | 0.24 |
| Peripheral vascular disease | 11.9% | 14.6% | 0.06 |
| Previous smoking | 51.0% | 62.4% | <0.001 ⁎ |
| Previous percutaneous coronary intervention | 36.1% | 43.0% | 0.001 ⁎ |
| Previous coronary bypass | 27.3% | 27.0% | 0.87 |
| Charlson index | 0.01 ⁎ | ||
| 0 | 48.0% | 42.9% | |
| 1 | 32.0% | 33.7% | |
| ≥2 | 20.0% | 23.4% | |
| Body mass index (kg/m 2 ) | 27.3 (24.3–31.6) | 27.8 (24.5–32.0) | 0.14 |
| Heart rate (beats/min) | 77 (66–90) | 74 (64–85) | <0.001 ⁎ |
| Systolic blood pressure (mm Hg) | 132 (116–149) | 135 (119–152) | 0.01 ⁎ |
| Serum sodium (mg/dl) | 139 (137–141) | 139 (137–141) | 0.01 ⁎ |
| Blood urea nitrogen (mg/dl) | 18 (14–25) | 17 (13–23) | <0.01 ⁎ |
| Serum creatinine (mg/dl) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 0.02 ⁎ |
| Hemoglobin (g/dl) | 13.5 (12.0–14.7) | 13.5 (12.2–14.7) | 0.58 |
| β-blocker use | 91.0% | 90.3% | 0.58 |
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker use | 86.0% | 82.6% | 0.03 ⁎ |
| Hydralazine use | 4.5% | 3.7% | 0.34 |
| Nitrate use | 14.2% | 18.7% | <0.01 ⁎ |
| Calcium channel blocker use | 27.8% | 29.7% | 0.32 |
| Aspirin use | 92.0% | 92.4% | 0.71 |
| Clopidogrel use | 46.4% | 52.9% | <0.01 ⁎ |
| Statin use | 74.9% | 76.6% | 0.33 |
| Diuretic use | 75.6% | 70.8% | 0.01 ⁎ |
† Cholesterol >200 mg/dl, low-density lipoprotein >130 mg/dl, high-density lipoprotein <30 mg/dl, or triglycerides >150 mg/dl.
Overall, 5-year survival for the study population was 58.3%. On unadjusted analysis, there were no significant differences between the event rates in those with and without AP for the end points of death; death or MI; death, MI, or revascularization; and death or hospitalization ( Table 2 ). Of note, most events for the revascularization composite occurred within the first 30 days of the index catheterization (30-day event rate 55% for the AP group vs 57% for the non-AP group). Those with AP were significantly more likely to reach the CV death or CV hospitalization end point compared with those without AP (p = 0.01).
| End point | Unadjusted Event Rate AP | p Value | Adjusted Event Rate AP | p Value | ||
|---|---|---|---|---|---|---|
| No | Yes | No | Yes | |||
| Death | 43% | 41% | 0.07 | 41% | 41% | 0.32 |
| Death or MI | 48% | 46% | 0.10 | 47% | 46% | 0.15 |
| Death, MI, or revascularization | 83% | 85% | 0.31 | 87% | 85% | 0.01 ⁎ |
| Death or hospitalization | 85% | 87% | 0.29 | 85% | 87% | 0.37 |
| CV death or CV hospitalization | 72% | 77% | 0.01 ⁎ | 73% | 77% | 0.03 ⁎ |
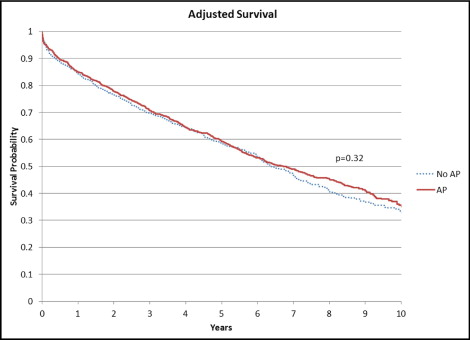
Variables that were significant in the unadjusted analysis or thought to have clinical relevance were included in multivariate models (see Table 2 footnote). Following risk adjustment, patients with and without AP had similarly high event rates for death ( Figure 2 ) as well as the composites of death or MI and death, or rehospitalization ( Table 2 ). Patients with AP had significantly higher rates of CV death or CV hospitalization (p = 0.03) but significantly lower rates of the composite end point of death, MI, or revascularization (p = 0.01) ( Table 2 , Figures 3 and 4 ). AP was an independent predictor of the revascularization composite and the CV death or CV hospitalization end point ( Table 3 ).