Coarctation of the Aorta
Karl F. Welke
Ross M. Ungerleider
Coarctation of the aorta can present as a severe and emergent problem in a neonate or as a subtle and essentially asymptomatic problem in an older child (or adult). This chapter focuses primarily on coarctation in neonates or infants because it is in that population that many of the important issues of coarctation are highlighted and best appreciated. In addition, this is the population that is most frequently treated with surgical repair of coarctation of the aorta. Between January 2007 and December 2010, 75% of the 3,667 patients who underwent surgical repair of coarctation of the aorta as recorded in the Society of Thoracic Surgeons Congenital Heart Surgery Database were under 1 year of age. Sixty-three percent of these patients were 30 days of age or younger.
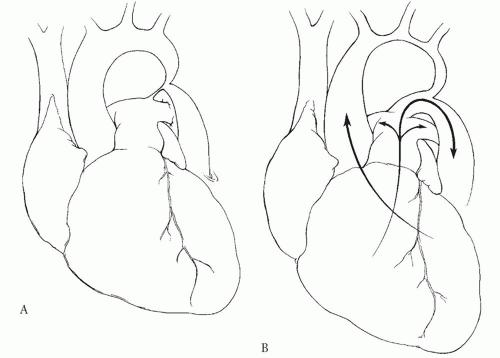
Coarctation is a form of left ventricular outflow tract obstruction (LVOTO) and imposes an increase in afterload to the left ventricle. It is often but not necessarily found in association with a variety of other important cardiac defects, and these can have a crucial effect on the physiology of the defect and on the patient’s presentation. In its “pure” form, coarctation is simply a constriction, or narrowing, of the aorta that usually occurs near the site of insertion of the ductus arteriosus. Because of this typical location, coarctation is often described as being “juxtaductal,” and this term is used to distinguish the more common forms of coarctation from a less common form that can involve the aorta proximal to the ductus arteriosus and extend into the transverse aortic arch. This latter type is sometimes referred to as “preductal” coarctation, and because it will usually present early in infancy, it can also be referred to as “infantile coarctation.” In practice, the use of the terms “juxtaductal” and “preductal” is not of critical importance as long as the extent of the coarctation is appreciated by the surgeon (Fig. 82.1).
Because of the obstruction to left ventricular (LV) outflow, as well as to distal aortic flow that is created by the coarctation, infants will generally suffer from severe LV failure manifested by poor distal perfusion and tachypnea. The LV outflow obstruction commonly results in pulmonary hypertension, and therefore “secondary” right ventricular (RV) hypertrophy is common in newborns with severe aortic coarctation. The LV failure may be reflected by a dilated, hypocontractile left ventricle with reduced output, and for this reason, the gradient across the coarctation site is not an indicator of the severity of the defect. The RV and LV features may be nicely demonstrated by transthoracic echocardiography. Furthermore, a wellperformed two-dimensional echocardiogram will demonstrate the anatomy of the aortic arch and the great vessels and the discrete area of aortic narrowing near the ductus. Flow to the distal aorta can be severely restricted, and patency of the ductus arteriosus is often essential in the newborn to preserve perfusion to the lower body (Fig. 82.1). Therefore, neonates with coarctation should be started on an intravenous infusion of prostaglandin E1 (PGE1). Maintaining ductal patency with PGE1 infusion will also allow decompression of the pulmonary circulation resulting from downstream obstruction of the LV. In neonatal coarctation, the RV may provide most of the perfusion in the descending aorta, and because the RV pressure may equal systemic pressure (especially if there is an associated ventricular septal defect [VSD]), there can be essentially no difference in the pressure above and below the coarctation. Therefore, the pressure gradient between the upper and the lower body will underestimate the severity of the coarctation. If no VSD is present, the descending aorta may be perfused with systemic venous blood from the RV and thereby results in the “differential cyanosis” that has been described for this lesion, with the lower body appearing more cyanotic than the upper body.
It is important to look for other commonly associated defects, which can occur anywhere along the “left heart/aorta complex.” A bicuspid aortic valve is present in over 50% of patients with coarctation of the aorta. Other associated defects include mitral stenosis (often with mitral anomaly such as single papillary muscle), hypoplastic left ventricle (defined as an LV volume of <20 ml/m2), endocardial fibroelastosis (a generalized scarring of the LV endothelium that appears as “brightness” on echocardiographic examination and that probably represents LV subendocardial ischemia), VSD (often with posterior malalignment of the infundibular septum that narrows the subaortic area), aortic stenosis (valvar or subvalvar), and atrial septal defect (ASD). All of these defects can be recognized by echocardiography, and it is not usually necessary to perform cardiac catheterization in these critically ill infants. The severity of the problem and the long-term prognosis are related to (1) age (young age, such as newborn, increases the risk), (2) the number and extent of associated defects, and (3) the actual anatomy of the defect (greater risk is incurred in patients whose defect extends proximal to the left subclavian artery).
Initial management of the newborn patient with aortic coarctation requires improving distal perfusion to the lower body by restoring the patency of the ductus arteriosus with an intravenous PGE1 infusion. Intubation and mechanical ventilation may be necessary due to the 15% to 20% incidence of apnea that occurs with PGE1 infusion. Once the patient has been stabilized, complete diagnostic assessment can be accomplished. This can be usually limited to an echocardiogram, which should demonstrate the anatomy of the aortic arch and isthmus, patency of the ductus arteriosus, the coarctation segment, and any important associated cardiac defects. It is not necessary to perform a cardiac catheterization for diagnostic purposes, but catheterization (or other form of imaging) should be done when there is any question about the arch anatomy, the nature of the coarctation, or the significance of a related defect that might require concomitant repair. Furthermore, cardiac catheterization has a
role when an intervention is desired before coarctation repair (such as a Rashkind atrial septostomy in patients with associated transposition of the great arteries who are not candidates for proceeding directly to the operating room). Operative repair should be considered once the diagnosis is confirmed. Coarctation of the aorta is an urgent problem in neonates, and prolonged medical management has a limited role reserved for unusual circumstances. There are currently several operative techniques for repair of aortic coarctation. Each has advantages and disadvantages, and the surgeon should be knowledgeable about each of these options. In most instances, repair is most easily and satisfactorily accomplished through a left thoracotomy, but occasionally median sternotomy is a useful approach and is discussed later.
role when an intervention is desired before coarctation repair (such as a Rashkind atrial septostomy in patients with associated transposition of the great arteries who are not candidates for proceeding directly to the operating room). Operative repair should be considered once the diagnosis is confirmed. Coarctation of the aorta is an urgent problem in neonates, and prolonged medical management has a limited role reserved for unusual circumstances. There are currently several operative techniques for repair of aortic coarctation. Each has advantages and disadvantages, and the surgeon should be knowledgeable about each of these options. In most instances, repair is most easily and satisfactorily accomplished through a left thoracotomy, but occasionally median sternotomy is a useful approach and is discussed later.
SURGICAL APPROACH: GENERAL CONSIDERATIONS
The majority of coarctations can be exposed and repaired through a left lateral thoracotomy. Preferably, an arterial monitoring line should be placed in the right radial artery. Occasionally the right radial artery is the end vessel of an aberrant right subclavian artery, in which case, it will not provide accurate pressure data during clamping of the aorta. The left radial artery is less desirable as the flow to the left upper extremity will be compromised by clamping of the left subclavian artery during the repair. An additional arterial monitoring line or a blood pressure cuff can be placed on a lower extremity. A pulse oximetry monitor should be placed on a lower extremity as well. The patient is placed in the right lateral decubitus position and prepped for a left lateral, muscle-sparing thoracotomy. As visualization is mostly needed posteriorly, the fourth intercostal space can be exposed via the auscultatory triangle. If additional exposure is needed, the serratus anterior muscle can be divided. The lung is retracted anteriorly and the mediastinal pleura is opened over the area of coarctation and retracted with stay sutures. Alternatively, the aorta can be approached via an extrapleural dissection. The vagus and left recurrent laryngeal nerves are then identified. The extent of the dissection of the aorta and its branches depends on the type of repair to be performed. Specifics will be discussed in the sections below. The ductus arteriosus can be encircled and securely tied just before or after the vascular clamps are placed on the proximal and distal aorta. Most patients, even infants, can have several collateral vessels arising from the aorta near the coarctation site. A Satinsky-type clamp on the descending aorta is useful to control posterior collaterals. Additional collaterals can be controlled by a variety of techniques. Small hemoclips can be placed on individual collaterals and then removed after coarctation repair. Alternatively, gold (temporary) Yasargil cerebral aneurysm clips can be used to control collaterals. These atraumatic clips can be placed to lay out of the way of the repair and easily removed at the conclusion of the operation. Proximal control can be obtained with a C-clamp that is applied to include the distal aortic arch and left subclavian artery. Distal control can be obtained with a Satinsky-type clamp as noted above or if preferred a straight or angled Debakey clamp. We do not routinely give heparin as it is unnecessary in the absence of atherosclerotic disease; however, some surgeons administer a low or moderate dose of heparin prior to clamp placement. After the clamps have been placed and the ductus has been ligated, we have found it helpful to aspirate the isolated segment of the aorta using a 22-gauge needle on a 3 ml syringe. If this portion of the aorta remains decompressed following evacuation of blood, the operation proceeds. If the aorta re-distends, a search is made for additional collateral vessels.
At the conclusion of the repair, the distal clamp is removed first to allow for deairing and initial inspection of the suture line. After alerting the anesthesiologist due to the possibility of transient hypotension, the proximal clamp is removed. At this point, pulsatile lower extremity flow should be detected by pulse oximetry. There may be an initial gradient between the upper and lower extremity blood pressures due to lower extremity vasoconstriction or to a Coanda effect that favors flow up of the innominate artery as opposed to around the transverse aortic arch; however, this should resolve within 30 minutes. The residual blood pressure gradient should be 10 mmHg or less. If there is concern about a persistently high gradient, direct measurement of aortic pressure above and below the repair may be useful. Closing the mediastinal pleura after end-to-end or subclavian flap repair depends on personal preference. Some believe that closing the mediastinal pleura over the repair can cause compression of the repair site and thereby increase the incidence of recurrent coarctation. Others believe that closing the mediastinal pleura after coarctation repair may prevent the lung from adhering to the repair site, decrease the incidence of postoperative chylothorax, and create a circumferential wrap around the repair site, which will make balloon dilation safer if recurrent coarctation occurs. After patch aortoplasty repair, closing the mediastinal pleura over the repair may not be possible,
and it may cause compression of the newly enlarged aorta and produce a suboptimal outcome.
and it may cause compression of the newly enlarged aorta and produce a suboptimal outcome.
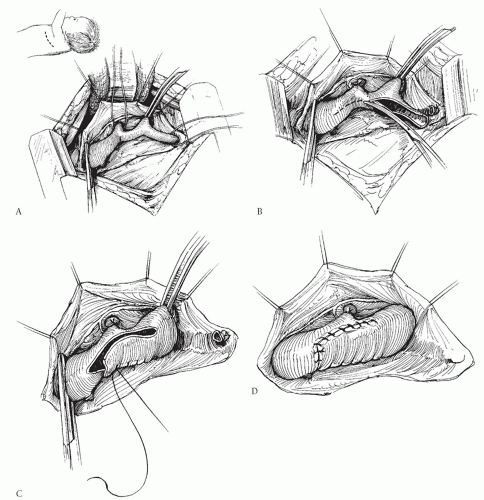
SUBCLAVIAN FLAP REPAIR
The technique of subclavian flap repair was once considered by many authorities to be the procedure of choice for neonates and infants. However, we do not favor it and recommend against its routine use. The technique requires division of the subclavian artery and turning it down as a flap to augment the area of coarctation (Fig. 82.2). Those who favor this procedure believe that it is simple and safe, and that the patch of subclavian artery will grow with the patient and therefore will lead to lower incidence of recurrent coarctation or late aneurysm formation. Unfortunately, recurrent coarctation does occur after this procedure with about the same incidence as after other commonly used procedures, and late aneurysm development has also been reported after this procedure. The subclavian flap procedure has the disadvantage that it requires permanent division of the left subclavian artery, and although this may be well tolerated in most patients, it can lead to long-term weakness of the left arm. Subclavian steal phenomenon has been described if the vertebral artery is left intact on the distal subclavian segment. Division of the left subclavian artery is problematic in the occasional patient with anomalous origin of the right subclavian artery below the coarctation site because in these patients, permanent loss of
the left subclavian artery leaves no way to follow pressures above the coarctation site. Although some have suggested techniques for reimplanting the left subclavian as an augmentation patch without division and with relief of more proximal problems, these procedures are cumbersome, unnecessary, and less attractive than alternatives. Finally, and importantly, this procedure does not address hypoplasia of the aortic arch that is proximal to the left subclavian artery—a frequent finding in neonatal coarctation.
the left subclavian artery leaves no way to follow pressures above the coarctation site. Although some have suggested techniques for reimplanting the left subclavian as an augmentation patch without division and with relief of more proximal problems, these procedures are cumbersome, unnecessary, and less attractive than alternatives. Finally, and importantly, this procedure does not address hypoplasia of the aortic arch that is proximal to the left subclavian artery—a frequent finding in neonatal coarctation.
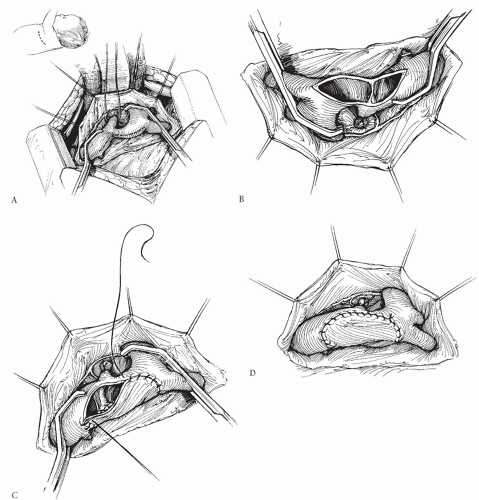
PATCH AORTOPLASTY
Several groups have advocated repair of discrete coarctation using a large patch of prosthetic material like Dacron, polytetrafluoroethylene (PTFE; Gore-Tex), or cryopreserved homograft (Fig. 82.3). Unlike the subclavian flap procedure, this technique does not require division of the subclavian artery, and the patch can be much larger than the subclavian flap patch. Furthermore, the patch can be extended proximally onto the aortic arch when necessary. Late aneurysm formation opposite the patch
has been reported by some, and this has tempered enthusiasm for this technique. More recent studies have suggested that the occurrence of these aneurysms may be related to easily controlled technical factors such as the type of prosthetic material used and to how the coarctation ridge is managed. Most late aneurysms have been associated with the use of Dacron as compared with PTFE. Avoiding resection of the coarctation shelf has also contributed to reducing the incidence of this complication. Using a large patch of PTFE will compensate for the relatively minimal intrusion of the posterior ridge into the aortic lumen. Patch aortoplasty has also been described using a technique in which the coarctation tissue is resected and the back wall of the aorta is anastomosed, with a patch then placed over the anterior wall. However, this more complicated technique may not be justified, considering the good results with simpler patch aortoplasty without resection of the coarctation shelf. The recurrence rate after patch aortoplasty is very low, and this remains a very acceptable option for some patients. It is especially useful for recurrent coarctation, or in adult-sized patients, when mobilization of the aorta is limited.
has been reported by some, and this has tempered enthusiasm for this technique. More recent studies have suggested that the occurrence of these aneurysms may be related to easily controlled technical factors such as the type of prosthetic material used and to how the coarctation ridge is managed. Most late aneurysms have been associated with the use of Dacron as compared with PTFE. Avoiding resection of the coarctation shelf has also contributed to reducing the incidence of this complication. Using a large patch of PTFE will compensate for the relatively minimal intrusion of the posterior ridge into the aortic lumen. Patch aortoplasty has also been described using a technique in which the coarctation tissue is resected and the back wall of the aorta is anastomosed, with a patch then placed over the anterior wall. However, this more complicated technique may not be justified, considering the good results with simpler patch aortoplasty without resection of the coarctation shelf. The recurrence rate after patch aortoplasty is very low, and this remains a very acceptable option for some patients. It is especially useful for recurrent coarctation, or in adult-sized patients, when mobilization of the aorta is limited.
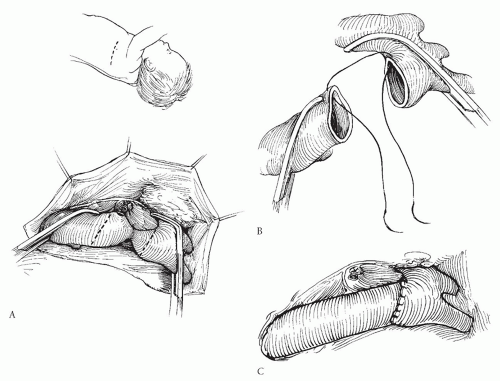
END-TO-END AND EXTENDED END-TO-END ANASTOMOSES
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree