Evidence of metabolic acidosis in fetal umbilical artery blood at birth (pH <7.00 and base deficit >12 mmol/L)
Early onset of severe or moderate encephalopathy in infants born at or beyond 34 weeks’ gestation
Cerebral palsy of the spastic quadriplegic or dyskinetic type
Exclusion of other identifiable etiologies such as trauma, coagulation disorders, infectious conditions, or genetic disorders (4)
progression of normoxia to hypoxia to metabolic acidosis to asphyxic damage and ultimately death is believed to occur in that order (Fig. 8.1). When the FHR pattern suggests hypoxia, all measures short of operative delivery (hydration, position change, supplemental oxygen, decreasing/discontinuing oxytocin, etc.) should be used to try to reverse the situation. If the hypoxic pattern cannot be reversed, then the fetus should be delivered expeditious. Clinical judgment needs to be stressed since if birth occurs because the fetus is thought to be hypoxic but not acidotic, then far too many unnecessary operative deliveries will be done, for the majority of hypoxic babies do not become acidotic, are vigorous at birth, and have normal outcomes.
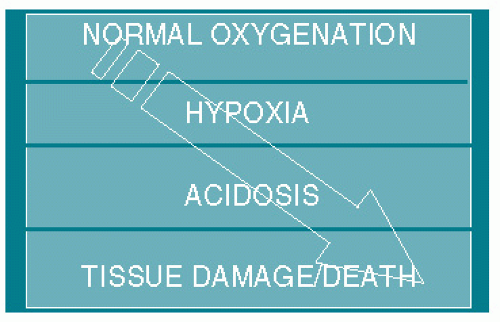 Figure 8.1. Model for declining fetal oxygenation with progressive development of hypoxia, metabolic acidosis, damage, and death. |
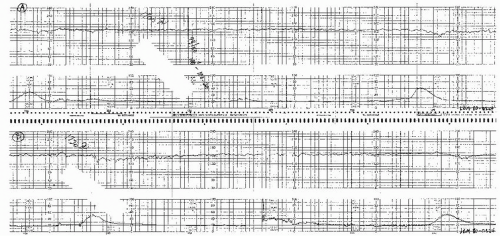
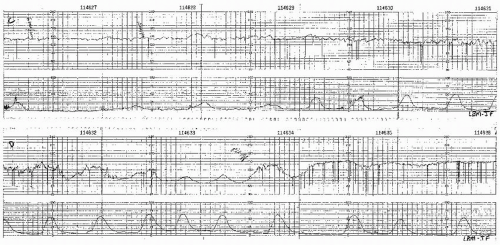
Visually apparent usually symmetrical gradual decrease and return of the FHR associated with a uterine contraction
A gradual FHR decrease is defined as from the onset to the FHR nadir of 30 seconds or more.
The decrease in FHR is calculated from the onset to the nadir of the deceleration.
The deceleration is delayed in timing, with the nadir of the deceleration occurring after the peak of the contraction.
In most cases, the onset, nadir, and recovery of the deceleration occur after the beginning, peak, and ending of the contraction, respectively.
and fetal scalp blood sampling is rarely performed. As long as the late decelerations persist and the pattern unchanged, efforts to rule out acidosis must be repeated at least every 30 minutes. The presence of repeated accelerations allows the clinician to follow such patterns as long as the labor is progressing satisfactorily.
TABLE 8.1 Medical management of late deceleration | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||
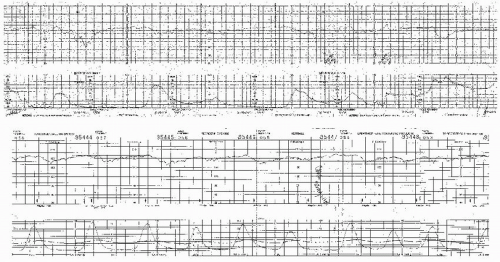
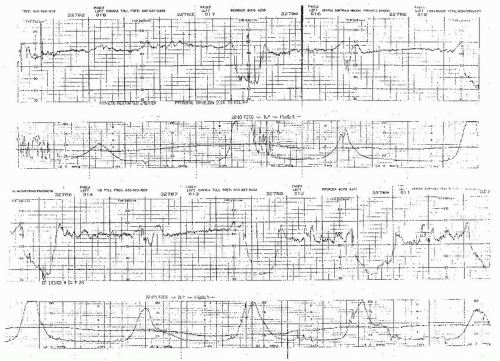
Visually apparent abrupt decrease in FHR
An abrupt FHR decrease is defined as from the onset of the deceleration to the beginning of the FHR nadir of <30 seconds.
The decrease in FHR is calculated from the onset to the nadir of the deceleration.
The decrease in FHR is 15 beats per minute (BPM) or greater, lasting 15 seconds or greater, and <2 minutes in duration.
When variable decelerations are associated with uterine contractions, their onset, depth, and duration commonly vary with successive uterine contractions.
The FHR decelerations last no more than 40 to 60 seconds on a repetitive basis.
The return of the FHR to the baseline is abrupt. There is no persistent “late component” manifested by a slow return or a late deceleration after the return.
The baseline FHR is not increasing.
The FHR variability is not decreasing.
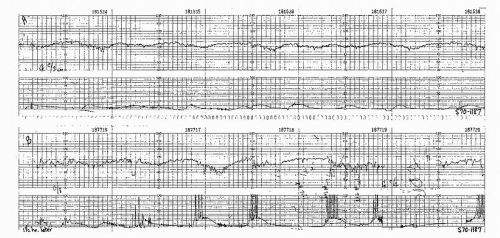
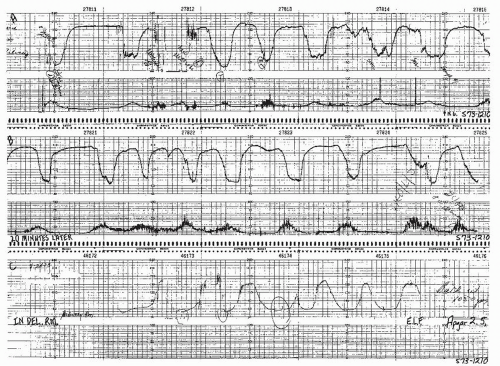
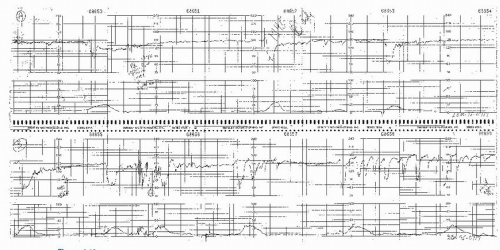 Figure 8.10. Variable deceleration with a rising baseline and slow return to baseline. This is an abnormal pattern. |
TABLE 8.2 Management classification of variable declarations | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
will be the threshold for intervention (Table 8.3). With the introduction of both prophylactic and therapeutic amnioinfusion, the frequency of variable decelerations should be decreased, often making management decisions much easier. Variable decelerations are responsible for most unnecessary cesarean sections performed when the physician is not experienced with fetal monitoring, and they must have been a major reason for operative intervention when only auscultatory FHR monitoring was available.
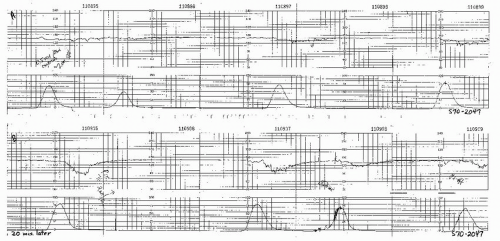
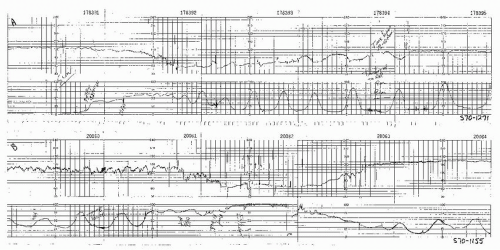
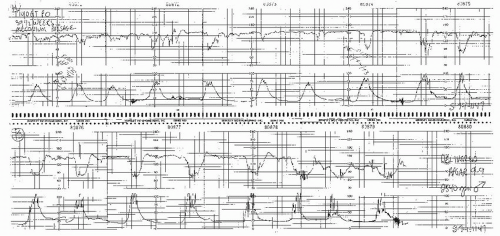 Figure 8.15. Variable deceleration of progressive severity occurring at the end of the second stage of labor. |
TABLE 8.3 Medical management of severe variable decelerations | ||||||
|---|---|---|---|---|---|---|
|
Visually apparent decrease in the FHR below the baseline
Decrease in FHR from the baseline that is 15 BPM or more, lasting 2 minutes or more but <10 minutes in duration
If a deceleration lasts 10 minutes or longer, it is a baseline change.
Hypertonic or prolonged contractions (spontaneous or oxytocin-induced) (Fig. 8.16)
Vaginal examination (Fig. 8.17)
Application of internal fetal scalp electrode
Fetal scalp blood sampling
Prolapsed umbilical cord (Fig. 8.18)
Maternal seizure
Epidural or spinal block (Fig. 8.19)
Supine hypotension
Fetal CNS anomalies
Prolonged umbilical cord compression, often associated with rapid descent of the fetus
Maternal respiratory arrest (high spinal, intravenous [IV] narcotic) or cardiac decompensation
Uterine rupture (Fig. 8.20)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree