The objective was to examine whether previous coronary artery disease (CAD) influences the add-on effects of the angiotensin II receptor blocker (ARB) valsartan on cardio-cerebrovascular morbidity and mortality in high-risk hypertensive patients who participated in the Kyoto Heart Study. The primary end point was the same as in the main study: a composite of new-onset and/or worsening of cardiovascular and cerebrovascular events. Median follow-up was 3.27 years. According to the presence of previous CAD at baseline, the study population was divided into 2 groups (with CAD, n = 707; without CAD, n = 2,324) in which primary end-point events occurred more frequently in patients with CAD than in patients without CAD (15.1% vs 5.6%, hazard ratio [HR] 2.68, 95% confidence interval [CI] 2.11 to 3.42). Add-on valsartan significantly decreased the occurrence of the primary end-point events in patients with CAD (11.3% vs 19.0%, HR 0.59, 95% CI 0.41 to 0.85) and without CAD (3.7% vs 7.6%, HR 0.49, 95% CI 0.34 to 0.70) compared to non-ARB treatment. In the presence of previous CAD, patients with valsartan add-on treatment had a significantly lower prevalence of angina pectoris and stroke than those with non-ARB treatment, whereas the valsartan add-on effects on angina and stroke were not significant in the absence of CAD. Changes in blood pressure during the follow-up period did not differ significantly between study subgroups. In conclusion, in the presence or absence of previous CAD, valsartan add-on treatment prevented more cardio-cerebrovascular events than conventional non-ARB treatment in high-risk hypertensive patients. In addition, valsartan add-on treatment conferred not only an antianginal effect but also stroke prevention exclusively in hypertensive patients with CAD compared to those without CAD.
The Kyoto Heart Study has demonstrated that angiotensin II receptor blocker (ARB) valsartan add-on treatment prevents more cardiovascular and cerebrovascular events than conventional non-ARB treatment in high-risk hypertensive patients. The purpose of the present post hoc analysis therefore was to examine whether the presence of previous coronary artery disease (CAD) influences the valsartan add-on effects on cardio-cerebrovascular morbidity and mortality in high-risk hypertensive patients using data from the Kyoto Heart Study.
Methods
The Kyoto Heart Study was designed as a multicenter, prospective, randomized, open-labeled, blinded end-point, 2-arm parallel treatment group comparison study with a response-dependent dose titration to evaluate the efficacy of ARB valsartan add-on treatment and conventional non-ARB treatment for decreasing cardio-cerebrovascular morbidity and mortality in high-risk hypertensive patients. The detailed design, organization, clinical measurements, and end-point definitions of the Kyoto Heart Study have been previously reported. Briefly, we recruited 3,031 Japanese high-risk patients with uncontrolled hypertension from hospitals collaborating with the Kyoto Prefectural University School of Medicine led by cardiology specialists from January 2004 through June 2007. Uncontrolled hypertension was defined as a mean sitting systolic blood pressure ≥140 mm Hg and/or a mean sitting diastolic blood pressure ≥90 mm Hg at 2 consecutive measurements in the outpatient clinic. High risk was defined as the presence of ≥1 of the following factors: CAD (angina pectoris or history of myocardial infarction >6 months before screening), cerebrovascular diseases (history of stroke or transient ischemic attack >6 months before screening), peripheral arterial occlusive disease, and/or ≥1 of the following cardiovascular risk factors and none of the exclusion criteria. Cardiovascular risk factors included type 2 diabetes mellitus, current smoking, dyslipidemia, obesity (body mass index ≥25 kg/m 2 ), and/or left ventricular hypertrophy defined by electrocardiography. Exclusion criteria were treatment with ARB before randomization or history of worsening heart failure, unstable angina, myocardial infarction, percutaneous coronary intervention, or coronary artery bypass grafting within the preceding 6 months. Detailed exclusion criteria of the Kyoto Heart Study have been previously reported. The protocol was approved by the ethics committee at each participating hospital and written consent was obtained from each patient.
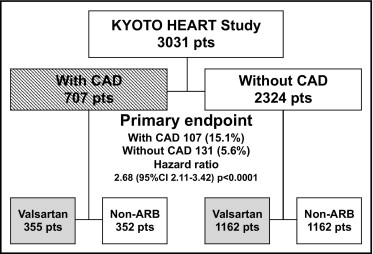
After confirmation, eligible patients were randomly assigned in accordance with the minimization method using 8 factors (age, gender, dyslipidemia, diabetes mellitus, smoking, obesity, history of CAD and/or cerebrovascular disease, and history of congestive heart failure) to the valsartan add-on group or to the conventional non-ARB treatment group. For the valsartan add-on group, valsartan 80 mg/day was administered as an initial dose and the dose was doubled after 4 weeks if the initial dose could not achieve the target blood pressure of <140/90 mm Hg. After 8 weeks an additional administration of other antihypertensive drugs except for ARBs and angiotensin-converting enzyme (ACE) inhibitors was allowed. For the conventional treatment group, antihypertensive drugs excluding ARBs and ACE inhibitors were provided. The detailed titration schedule has been previously described. In the present post hoc analysis, according to the presence of previous CAD at baseline, the Kyoto Heart Study population was divided into 2 groups (with CAD, n = 707; without CAD, n = 2,324; Figure 1 ) . Periodic follow-up examinations were performed every 6 months after setting the durable dose. Study measurements and data management were described in the design paper.
The primary end point in this post hoc analysis was the same as in the main study: new-onset and/or worsening of cardiovascular and cerebrovascular events. They included the following events: stroke (hospitalization and diagnosed by computed tomography and/or magnetic resonance imaging), new or recurrent transient ischemic attack (hospitalization and diagnosed by computed tomography and/or magnetic resonance imaging and sudden onset of a neurologic deficit persisting <24 hours without a history of atrial arrhythmia causing the embolism), new or recurrent acute myocardial infarction (hospitalization, electrocardiographic change, and biomarkers for myocardial infarction), new occurrence or exacerbation of angina pectoris (hospitalization and diagnosed by electrocardiographic changes coinciding with chest symptoms and coronary angiogram showing >75% stenosis according to American Heart Association/American College of Cardiology guidelines), new occurrence or exacerbation of heart failure (hospitalization and clinical symptoms concomitant with left ventricular dysfunction by echocardiography according to American Heart Association/American College of Cardiology guidelines), dissecting aneurysm of the aorta (hospitalization and diagnosed by an imaging technique), lower limb arterial obstruction, emergency thrombosis, transition to dialysis, and doubling of plasma creatine levels. Secondary end points included all-cause mortality, worsening of cardiac function, new occurrence or exacerbation of arrhythmias, new occurrence or exacerbation of diabetes mellitus or impaired glucose tolerance, and uncontrolled blood pressure. The event evaluation was performed independently by the end-point committee.
All continues data are expressed as mean ± SD. Baseline characteristics were compared between subgroups using Student’s 2-sample 2-sided test or Mann–Whitney test. Fisher’s exact test or chi-square test was used for categorical variables. Analysis of change in blood pressure was performed with STATA (STATA Corp., College Station, Texas) using 2-way analysis of variance with repeated measurements. All time-to-event variables were displayed using the Kaplan–Meier estimate according to groups. Event rates were adjusted for gender, age, diabetes, smoking, dyslipidemia, obesity, statin, and concomitant antihypertensive treatment, and Cox proportional hazard regression analysis was used to compare event rates between groups. All tests were 2-sided. A p value <0.05 was considered statistically significance.
Results
Baseline characteristics for all 3,031 patients are presented in Table 1 . During the median follow-up of 3.27 years, 18 patients in the valsartan add-on arm and 16 patients in the conventional non-ARB arm were lost because of consent withdrawal or loss of follow-up. In the present post hoc analysis, the study population was divided into 2 groups according to the presence of previous CAD and then divided into valsartan add-on and non-ARB treatments, respectively ( Figure 1 ). At baseline overall, patients with CAD were older age, more frequently men, and had lower diastolic blood pressure, lower left ventricular ejection fraction, lower values of low-density lipoprotein, high-density lipoprotein, triglyceride, and estimated glomerular filtration rate, a higher prevalence of diabetes mellitus, and a lower prevalence of obesity (body mass index ≥25) than those without CAD ( Table 1 ). In the presence or absence of CAD, baseline clinical characteristics did not differ significantly between patients with valsartan add-on treatment and patients with conventional non-ARB treatment ( Table 1 ). Baseline medications before randomization are listed in Table 2 . Overall, patients with CAD had greater usage of calcium channel blockers, ACE inhibitors, β-blockers, diuretics, and statins than those without CAD. In the presence or absence of CAD, the distribution of baseline medications did not differ significantly between patients with valsartan add-on treatment and patients with conventional non-ARB treatment.
| Characteristics | With CAD | Without CAD | With vs Without CAD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 707) | Valsartan (n = 355) | Non-ARB (n = 352) | p Value | All (n = 2,324) | Valsartan (n = 1,162) | Non-ARB (n = 1,162) | p Value | ||
| Age (years) | 70 ± 9 | 70 ± 9 | 70 ± 9 | 0.3001 | 65 ± 11 | 65 ± 11 | 65 ± 11 | 0.6964 | <0.0001 |
| Men/women | 475/232 | 236/119 | 239/113 | 0.7477 | 1,253/1,071 | 625/537 | 628/534 | 0.9337 | 0.0001 |
| Systolic blood pressure (mm Hg) | 156 ± 14 | 157 ± 15 | 154 ± 13 | 0.1226 | 157 ± 14 | 157 ± 14 | 157 ± 14 | 0.6936 | 0.0959 |
| Diastolic blood pressure (mm Hg) | 85 ± 11 | 85 ± 11 | 85 ± 11 | 0.7651 | 89 ± 11 | 89 ± 11 | 89 ± 12 | 0.1549 | 0.0001 |
| Heart rate (beats/min) | 70 ± 15 | 70 ± 15 | 70 ± 15 | 0.8514 | 70 ± 18 | 70 ± 18 | 71 ± 17 | 0.1815 | 0.9772 |
| Body mass index (kg/cm 2 ) | 24 ± 3 | 24 ± 4 | 24 ± 3 | 0.7621 | 25 ± 4 | 25 ± 4 | 25 ± 4 | 0.5037 | 0.0522 |
| Waist size (cm) | 86 ± 10 | 86 ± 10 | 86 ± 10 | 0.7385 | 85 ± 11 | 86 ± 10 | 85 ± 11 | 0.5763 | 0.4057 |
| Cardiothoracic ratio (%) | 51 ± 6 | 50 ± 6 | 52 ± 6 | 0.1606 | 51 ± 5 | 51 ± 6 | 51 ± 5 | 0.6805 | 0.8049 |
| Electrocardiography (S wave in lead V 1 + R wave in lead V 5 , mm) | 30 ± 11 | 30 ± 11 | 30 ± 12 | 0.6086 | 31 ± 11 | 31 ± 10 | 30 ± 11 | 0.4892 | 0.2326 |
| Echocardiographic ejection fraction (%) | 60 ± 11 | 60 ± 11 | 60 ± 11 | 0.8230 | 64 ± 9 | 64 ± 9 | 64 ± 9 | 0.9215 | <0.0001 |
| Low-density lipoprotein cholesterol (mg/dl) | 113 ± 30 | 112 ± 30 | 115 ± 29 | 0.1448 | 125 ± 42 | 125 ± 51 | 125 ± 31 | 0.8562 | <0.0001 |
| High-density lipoprotein cholesterol (mg/dl) | 52 ± 15 | 53 ± 15 | 51 ± 15 | 0.1572 | 56 ± 16 | 56 ± 15 | 56 ± 16 | 0.7673 | <0.0001 |
| Triglycerides (mg/dl) | 144 ± 78 | 143 ± 80 | 144 ± 75 | 0.8137 | 153 ± 99 | 152 ± 100 | 155 ± 98 | 0.4060 | <0.0001 |
| Hemoglobin A1c (%) | 6.2 ± 2.5 | 6.2 ± 3.3 | 6.2 ± 1.3 | 0.9316 | 6.0 ± 1.7 | 6.1 ± 2.1 | 5.9 ± 1.2 | 0.1237 | 0.0361 |
| Fasting plasma glucose (mg/dl) | 123 ± 42 | 120 ± 40 | 125 ± 44 | 0.1332 | 122 ± 47 | 122 ± 49 | 121 ± 46 | 0.4399 | 0.5669 |
| Serum creatinine (mg/dl) | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.9 ± 0.3 | 0.2332 | 0.8 ± 0.3 | 0.8 ± 0.2 | 0.8 ± 0.3 | 0.7180 | 0.0777 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) | 64 ± 20 | 64 ± 20 | 63 ± 20 | 0.5389 | 70 ± 20 | 70 ± 19 | 70 ± 20 | 0.7773 | <0.0001 |
| Serum sodium (mEq/L) | 142 ± 17 | 141 ± 8 | 143 ± 22 | 0.1043 | 144 ± 83 | 143 ± 40 | 145 ± 110 | 0.5119 | 0.6242 |
| Serum potassium (mEq/L) | 4.2 ± 0.4 | 4.2 ± 0.4 | 4.2 ± 0.4 | 0.9832 | 4.5 ± 7.0 | 4.6 ± 9.6 | 4.3 ± 2.5 | 0.4293 | 0.4029 |
| Current smokers | 152 (21%) | 76 (21%) | 76 (22%) | 0.9741 | 521 (22%) | 265 (23%) | 256 (22%) | 0.6907 | 0.6433 |
| Obesity | 231 (33%) | 111 (31%) | 120 (35%) | 0.4715 | 946 (41%) | 482 (41%) | 464 (40%) | 0.4729 | 0.0001 |
| Diabetes mellitus | 212 (30%) | 98 (28%) | 114 (32%) | 0.1919 | 595 (26%) | 303 (26%) | 292 (25%) | 0.6346 | 0.0238 |
| Dyslipidemia | 484 (68%) | 236 (66%) | 248 (70%) | 0.2907 | 1,660 (71%) | 830 (71%) | 830 (71%) | 0.9634 | 0.1408 |
| Cerebrovascular disease | 23 (3%) | 11 (3%) | 12 (3%) | 0.9835 | 100 (4%) | 47 (4%) | 47 (4%) | 0.6093 | 0.2586 |
| Heart failure | 80 (11%) | 33 (9%) | 47 (13%) | 0.1133 | 113 (5%) | 51 (4%) | 62 (5%) | 0.3348 | 0.0666 |
| Medications | With CAD | Without CAD | With vs Without CAD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| All (n = 707) | Valsartan (n = 355) | Non-ARB (n = 352) | p Value | All (n = 2,324) | Valsartan (n = 1,162) | Non-ARB (n = 1,162) | p Value | ||
| Calcium channel blocker | 432 (61%) | 217 (61%) | 215 (61%) | 0.9488 | 1,225 (53%) | 608 (52%) | 617 (53%) | 0.7396 | 0.0001 |
| Angiotensin-converting enzyme inhibitor | 204 (29%) | 101 (28%) | 103 (29%) | 0.8769 | 390 (17%) | 188 (16%) | 202 (17%) | 0.4705 | <0.0001 |
| β Blocker | 224 (32%) | 109 (31%) | 115 (33%) | 0.6305 | 317 (14%) | 155 (13%) | 162 (14%) | 0.7169 | <0.0001 |
| α Blocker | 24 (3%) | 12 (3%) | 12 (3%) | 0.8520 | 72 (3%) | 33 (3%) | 39 (3%) | 0.5494 | 0.7859 |
| Thiazide | 19 (3%) | 9 (3%) | 10 (3%) | 0.9850 | 78 (3%) | 43 (4%) | 35 (3%) | 0.4201 | 0.4456 |
| Antialdosterone | 25 (4%) | 15 (4%) | 10 (3%) | 0.4278 | 32 (1%) | 16 (1%) | 16 (1%) | 0.8587 | 0.0004 |
| Other diuretics | 75 (11%) | 31 (9%) | 44 (13%) | 0.1325 | 87 (4%) | 45 (4%) | 42 (4%) | 0.8270 | <0.0001 |
| Statin | 351 (50%) | 167 (47%) | 184 (52%) | 0.1883 | 643 (28%) | 324 (28%) | 319 (27%) | 0.8529 | <0.0001 |
| Fibrate | 17 (2%) | 11 (3%) | 6 (2%) | 0.3349 | 48 (2%) | 24 (2%) | 24 (2%) | 0.8840 | 0.6915 |
| Other antihyperlipidemic agents | 26 (4%) | 14 (4%) | 12 (3%) | 0.8589 | 48 (2%) | 31 (3%) | 17 (1%) | 0.0580 | 0.0519 |
| Sulfonyl urea | 94 (13%) | 46 (13%) | 48 (14%) | 0.8769 | 253 (11%) | 128 (11%) | 125 (11%) | 0.8940 | 0.0902 |
| Other oral hyperglycemic agents | 81 (11%) | 37 (10%) | 44 (13%) | 0.4538 | 203 (9%) | 104 (9%) | 99 (9%) | 0.7689 | 0.0536 |
| Insulin | 31 (4%) | 12 (3%) | 19 (5%) | 0.2601 | 51 (2%) | 26 (2%) | 25 (2%) | 1.0000 | 0.0620 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


