Fig. 9.1
After opening of the pericardium, the aorta and pulmonary artery are isolated and dissected in preparation for division
Figure 9.2 shows the lines of transaction for the SVC, PA, and aorta. Heparin is administered at 300–400 IU/Kg following the dissection of the abdominal organs [9]. It is important to perform a short briefing session with the recovery teams present in order to avoid any misunderstanding before the initiation of the terminal recovery event and organs perfusion. If the lungs are also being procured, prostaglandin E1 is delivered at this time in the mid-pulmonary artery to reduce vasoconstriction caused by high-potassium concentration, although the efficacy of this remains controversial [10]. As hypotension ensues following the administration of prostaglandin, the inflow is eliminated by clamping the SVC and incising the anterior wall of IVC to allow free flow of the cardioplegia solution. If lungs are recovered, we place a clamp across the LA appendage and amputate it prior to application of cross-clamp. It is essential to make sure to make a large enough egress to allow free flow of the pulmoplegia and prevent LV distention. If the lungs are not recovered, transaction of either the right or left superior pulmonary vein (PV) can allow decompression of the left heart. The LA clamp is opened and the aorta is cross-clamped. Cardioplegia is initiated at 150 mmHg using pressure bags [9]. We use cold storage solution, as developed by F.O. Belzer and James Southard for cardioplegia and storage. This is available as Static Preservation Solution (SPS-1™, Organ Recovery Systems, Chicago, IL). This is cooled between 2 °C and 4 °C. Two liters are hung in pressure bags prior to cross-clamp. Topical cooling is accomplished with iced saline slush at 4°. It is important to avoid application of large ice blocks on the right ventricle (RV) since this may cause freezing injury to the RV. Once the infusion of preservation solution is complete, the SVC, aorta, and PA are transected along with the SVC.
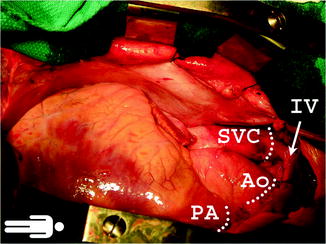

Fig. 9.2
The superior vena cava, pulmonary artery, and aorta shown after their dissection is complete
Figure 9.3 shows transaction of the SVC, aorta, and amputation of the LA appendage.
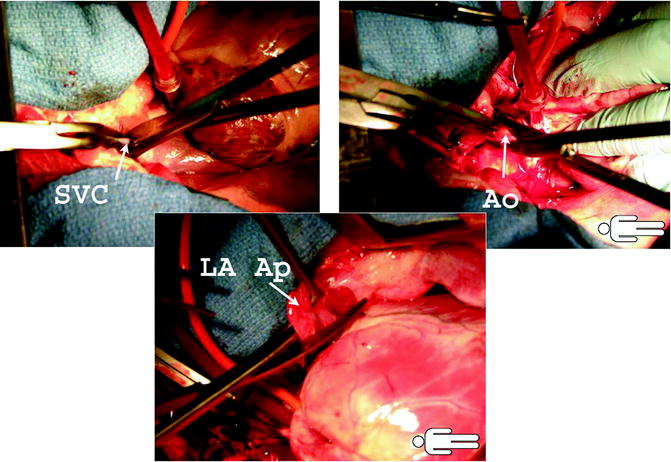

Fig. 9.3
The upper images demonstrate division of the superior vena cava and aorta. The lower images show amputation of the left atrial appendage. This is followed by division of the left atrium and pulmonary veins
We routinely leave the aortic vent and cardioplegia needle in for use in the recipient as an aortic de-airing needle. Lastly, the LA or PVs are divided. If lungs are not recovered, then division of right and pulmonary veins is expeditiously achieved. Judicious, left atrial division has to occur when lungs are also recovered. Firstly the Sondergaard’s groove has to be developed for at least ½ inch between the right atrium and left atrium in front of the right pulmonary veins. We do prefer to open the left atrium at this level leaving at least 1.5 cm of atrial cuff for the right pulmonary veins to be taken with the right lung.
On the back table in the donor OR room, a sterile bag is placed inside a rigid container and filled with SPS using a bag decanter. The donor heart is inspected for the presence of patent foramen ovale (PFO) and other possible injuries (shown in Fig. 9.4). The container is then placed inside two more sterile bags. The outside bag is labeled with a tag describing its contents and then immersed on ice in a transport cooler [9]. The label includes the organ-specific internal color-coded label provided by UNOS completed with the Organ Procurement and Transplantation Network (OPTN) donor I.D. number, donor ABO type, and description of the specific contents (i.e., heart).
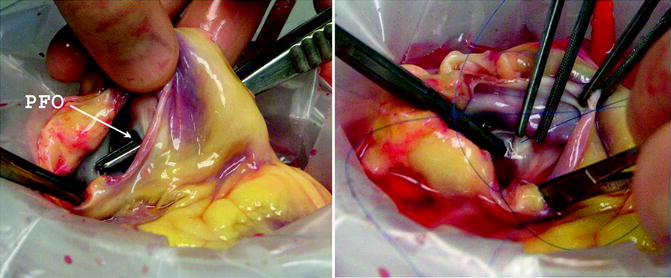

Fig. 9.4
If a patent foramen ovale is found, this is closed on the back table during preparation of the donor heart
Back Table Preparation of the Donor Heart
Back table preparation is typically undertaken at the removal of the transplant heart from the cooler upon arrival in the recipient operating room. When lung recovery did not occur, the LA is left intact, the PVs are opened from between the lower and upper veins on each side and then across [9, 11]. The excess tissue is trimmed in order to provide an atrial cuff to match that of the recipient LA. The atrial wall is inspected for a PFO, and if present this is oversewn [9], usually from the right atrium as depicted in Fig. 9.4.
Electrocautery is used to separate the PA and aorta along its length (Fig. 9.5). The valves are quickly visualized to ensure they were not damaged during the recovery process.
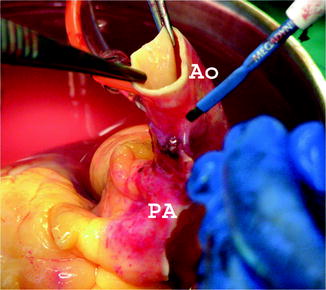

Fig. 9.5
The pulmonary artery and aorta are separated along their length on the back table
Figure 9.6 illustrates the potential injury to the back wall of the aorta with the cardioplegia needle.
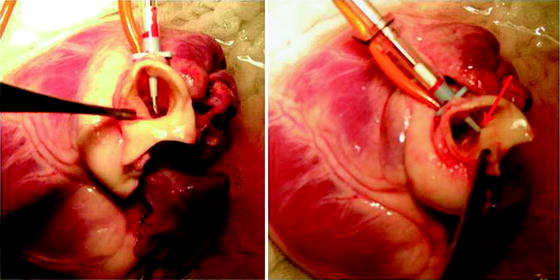

Fig. 9.6
Care must be taken to prevent and look for back wall injury to the aorta from the cardioplegia needle
If the venting of the heart was performed through the LA appendage, during heart procurement it should be closed with 4-0 single filament polypropylene running suture. This is illustrated in Fig. 9.7.
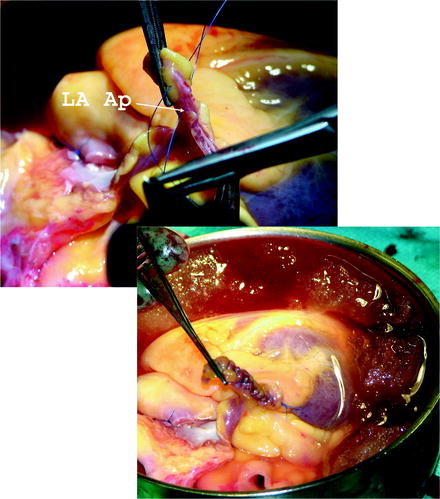

Fig. 9.7
The site of the left atrial appendage is closed on the back table using 4-0 monofilament suture
Recipient Cardiectomy
Standard preoperative antibiotics are administered. Adequate intravenous access and invasive systemic and pulmonary arterial monitoring are established. The recipient operation is approached most of the time through a median sternotomy, including in re-operative procedures. Occasionally, axillary or femoral cannulation is performed. Sternal adhesions may significantly increase the risk of complications on re-operation [12]. To ensure an easier access for the future redo sternotomy, we routinely reconstruct the pericardium with 2-mm-thick Gore-Tex patch (Gore, Flagstaff, Arizona) at our institution, after left ventricular assist device (LVAD) implantation as bridge to transplantation (Fig. 9.8). We found that this significantly increases the ease of the subsequent sternotomy.
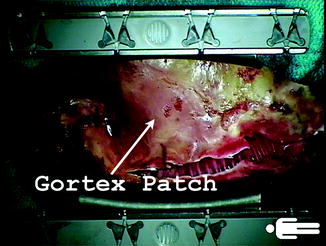

Fig. 9.8
A 2-mm-thick Gore-Tex patch used to reconstruct the pericardium at the time of left ventricular assist device implantation is of great help during the subsequent sternotomy at the time of transplant
Low-flow carbon dioxide is insufflated into the operative field to reduce the risk of air embolism [13, 14]. The aorta is cannulated as distally as possible in the ascending aorta. The SVC and IVC are cannulated with right-angle 24–28 F and 28 F cannulas, respectively. Cardiopulmonary bypass is initiated and moderate hypothermia is employed.
Snares are placed around both SVC and IVC cannulae. The aorta is mobilized off of the PA to allow enough room for and safe placement of the aortic cross-clamp. This is particularly important in re-operative surgery when dense scarring may be present between the aorta and pulmonary artery. We initiate the terminal cardiectomy when we ensure that the donor team has safely landed. This allows us approximately 20 min for the recipient cardiectomy. This is initiated by tightening the snares around each vena cava followed by application of cross-clamp after temporarily lowering the flow on cardiopulmonary bypass. We divide the upper and lower parts of the right atrium contiguous with the SVC and IVC leaving a cuff of right atrium of at least 2–3 cm on each vena cava. Care is taken during this procedure to avoid injury of the left atrium especially with re-operative surgery. Preexisting pacing/defibrillator wires are pulled intrapericardially and cut flush with the SVC snare with a wire cutter. Swan–Ganz catheter is removed from the recipient heart at this point and retracted out of the mediastinum and secured to the drapes. Aorta and the PA are both divided at the level of the valve commissures. Lastly, the LA is divided along the atrioventricular groove. If an LVAD is present, the left ventricle can be amputated close to the apex and LVAD cannula oriented outside of the pericardial cavity and removed after completion of all anastomoses.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


