Atrial Septal Defects
Richard D. Mainwaring
Peter Pastuszko
John J. Lamberti
Substantial changes have occurred in the management of atrial septal defects (ASDs) since the approval of the Amplatzer ASD occluder device by the Food and Drug Administration in 2002. Today, the vast majority of isolated ASDs are closed in the cardiac catheterization laboratory. However, secundum-type ASDs are frequently a component of more complex congenital heart defects and need to be closed surgically as part of the procedure. Also, other forms of ASD would appear to remain in the surgical domain for at least the foreseeable future. Therefore, it is still imperative that surgeons performing pediatric heart operations understand the physiology of these defects, the indications for intervention, and the surgical techniques used for their repair.
Secundum-type ASDs account for approximately 80% to 85% of all ASDs. Sinus venosus defects (both the superior and the inferior type) and incomplete atrioventricular (AV) canal defects each constitute between 5% and 10% of all ASDs. Coronary sinus defects are a relatively uncommon form of ASD. The topic of incomplete AV canal—type ASDs is addressed in Chapter 82.
Secundum-type ASDs are three times more frequent in female than male patients. In addition, there is also a significant incidence of familial inheritance. These observations indicate that genetic factors play an important role in the formation of secundum ASDs; however, the exact gene locus responsible for this phenomenon has not been identified.
Most ASDs result in a left-to-right shunt (Qp:Qs) of about 2 or 3:1. The amount of flow across an ASD is determined by the relative ratio of diastolic compliance between the right and left ventricles because antegrade flow of the systemic and pulmonary venous return within the heart require the AV valves to be open. The amount of shunt is also influenced by the size of the interatrial communication, the presence or absence of pulmonary valve stenosis, and the pulmonary vascular resistance. Despite the increase in pulmonary blood flow seen with ASDs, most patients will have normal pulmonary artery (PA) pressures.
The chronic volume overload associated with an ASD has several adverse effects. The increase in flow through the left atrium (LA), right atrium (RA), and right ventricle (RV) results in enlargement of these structures. It is clear that cardiac enlargement, regardless of its etiology, will eventually translate into decreased quality of life as well as decreased life expectancy. With ASDs, the heart enlargement may take many decades before it leads to ventricular dysfunction or the onset of atrial arrhythmias. Similarly, the increase in pulmonary blood flow is initially well tolerated, but on a long-term basis may result in increased pulmonary vascular resistance.
The natural history of an ASD has been well documented. Studies indicate that patients with unrepaired ASDs and a Qp:Qs of >1.5:1 will have a diminished life expectancy, averaging about 45 years. There is a great deal of variability in the natural history of these patients. Most infants and young children with ASDs are asymptomatic, demonstrating normal growth and absence of cyanosis. Some school-age children will tire a bit more easily than their peers or siblings and occasionally demonstrate perioral or periorbital cyanosis following exertion. As patients with untreated ASDs enter into their teens and 20s, they may have a gradual decline in their exercise capacity. Also during this time, women enter into their childbearing years. Women with an untreated ASD who become pregnant have a significant incidence of fetal (20% to 30%) and maternal (2%) mortality. As patients enter into their 30s and 40s, most experience a progressive decline in their exercise tolerance coincident with the deterioration in right ventricular function. Atrial arrhythmias are usually a late occurrence and may be the cause of palpitations. The development of arrhythmias may tip a previously well-compensated patient into congestive heart failure because of the loss of atrial contraction. Cyanosis is usually a very late and ominous sign of irreversible pulmonary vascular disease. Patients with a right-to-left shunt or a minimal left-to-right shunt are at risk for paradoxical embolus, although the actual risk is difficult to quantitate. The combination of right heart failure, arrhythmias, and cyanosis generally progresses and eventually leads to the eventual demise of the patient.
 DIAGNOSIS
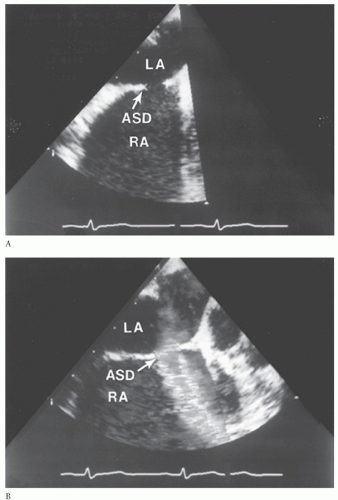
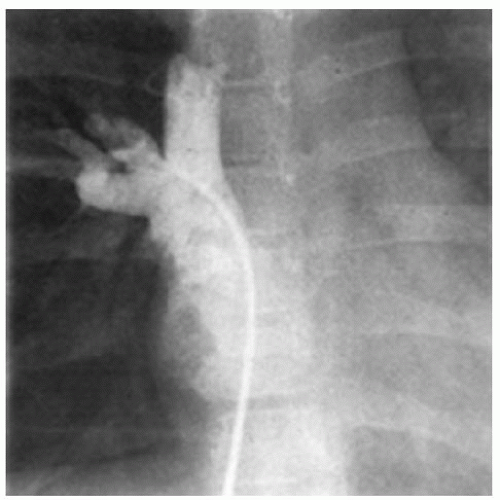
DIAGNOSISThe diagnosis of ASD is usually suspected based on the physical examination. The history may not be particularly helpful in children but is an important part of the assessment in adults. Echocardiography is used to confirm the diagnosis and to delineate the pulmonary venous drainage (Fig. 79.1). Echocardiographic diagnosis is sufficient in most cases because the vast majority of patients identified with ASDs are infants and children. Diagnostic cardiac catheterization is indicated in two specific circumstances. In children, the need for catheterization arises when there remains doubt as to the complete diagnosis (Fig. 79.2). This can occur with abnormalities of systemic or pulmonary venous drainage or to exclude other associated congenital heart defects. Diagnostic catheterization is indicated in adult patients to assess pulmonary vascular resistance and to exclude the presence of acquired heart disease.
RECOMMENDATION FOR CLOSURE OF ATRIAL SEPTAL DEFECTS
The recommendation for closure of ASDs is based on the premise that the risk of the intervention is substantially lower than the risk incurred by following the natural history. ASD repair prevents the progression of cardiac and pulmonary changes and in younger patients restores a normal life expectancy. When the diagnosis of ASD is
made in infancy or early childhood, closure is generally recommended between 2 and 4 years of age. Regardless of the technique selected (surgery vs. interventional catheterization), these procedures are usually well tolerated and are typically associated with a rapid recovery. The psychosocial aspects of the hospitalization may also be better handled at this age.
made in infancy or early childhood, closure is generally recommended between 2 and 4 years of age. Regardless of the technique selected (surgery vs. interventional catheterization), these procedures are usually well tolerated and are typically associated with a rapid recovery. The psychosocial aspects of the hospitalization may also be better handled at this age.
Patients with an ASD detected in adulthood will usually benefit from closure of the defect and remain good candidates for whichever technique is selected. Assessment of the adult patient is based on a combination of clinical history, physical findings, echocardiographic information, and cardiac catheterization data. Patients who are fully saturated (i.e., have a left-to-right shunt by echo) and have RV enlargement consistent with a volume load from the shunt will almost certainly derive considerable benefit from closure. Conversely, most patients who have developed cyanosis should not be considered for closure because they rarely benefit from this intervention. Cardiac catheterization may better define those patients in the gray zone. Pulmonary vascular resistance approaching systemic or a shunt ratio barely exceeding 1 suggests that the patient is not a candidate for closure (approximately 10% of patients by the fourth decade will have reached this stage). The conventional teaching has been that surgical intervention is warranted in patients with a Qp:Qs of >1.5:1, but this criterion can probably be lowered to a Qp:Qs of 1.2 or 1.3:1 with the less invasive septal occluder devices. Closure should also be strongly considered in patients with a history of stroke even if the defect is very small, in order to preclude subsequent events.
Women who are discovered to have an ASD during pregnancy represent a unique clinical situation. During pregnancy, the circulating blood volume expands by about 50%, allowing even more blood to shunt through the ASD. Women who were previously well compensated may develop signs and symptoms of heart failure during the third trimester of their pregnancy. Every attempt should be made to avoid cardiac surgery in this circumstance because the risk to both mother and fetus is quite high. Most pregnant women can be successfully
managed using a combination of medical therapy and bed rest. However, on occasion a patient cannot be stabilized and must undergo urgent intervention. This mode of presentation was more common years ago than it is today, presumably because of improved methods of diagnosis.
managed using a combination of medical therapy and bed rest. However, on occasion a patient cannot be stabilized and must undergo urgent intervention. This mode of presentation was more common years ago than it is today, presumably because of improved methods of diagnosis.
 SURGICAL TECHNIQUE
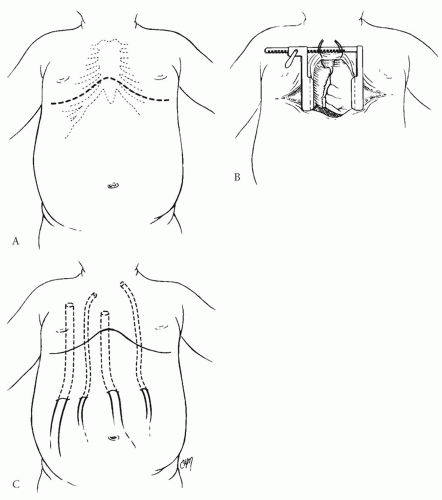
SURGICAL TECHNIQUEASDs are usually closed through a sternotomy approach. In boys, this can be performed through a relatively short, low-lying midline incision. Our preference in girls has been to use a curvilinear, transverse (inframammary) skin incision as shown in Figure 79.3. This technique, once it has been learned, provides a superior cosmetic result without compromising in any way the exposure or versatility. Independent of the orientation of the skin incision, the sternum is divided vertically through the midline. The sternal retractor is then positioned for exposure. The retractor does not need to be opened widely for this operation because visualization of the ASD is relatively straightforward. Excessive retraction can result in sternal fracture.
The thymus is a relatively large structure in younger children and can present an obstacle to cannulation of the aorta. A portion of the thymus can be excised to improve exposure. This maneuver is not necessary in adult patients. The pericardium is then opened according to the intended operation. For the repair of a secundum-type ASD, we prefer to open the pericardium slightly to the left of midline with the anticipation that the patch will be cut to the appropriate size and shape once the defect has been visualized. For the repair of a sinus venosus-type defect, the anterior pericardium can be harvested and preserved. Some surgeons prefer to treat the pericardium with glutaraldehyde because this both strengthens the patch and makes handling it a bit more straightforward. The remaining pericardial edges are sutured to the drapes to form a pericardial well. Inspection of the anatomy is then conducted with several specific considerations:
The heart is inspected with regard to its size and configuration. Often the degree of cardiac enlargement will seem disproportionate to that seen on chest X-ray film.
The great vessels are inspected and palpated. The PA is usually enlarged consistent with increased flow through this vessel. A thrill at the level of the pulmonary annulus may signify the presence of pulmonary stenosis.
The systemic veins are inspected to ensure that there are no abnormalities. A small or absent innominate vein suggests the presence of a left superior vena cava (LSVC). In the case of a superior sinus venosus defect, the SVC is isolated, and the presence or absence of anomalous pulmonary veins is confirmed. The position of the azygos vein should also be identified.
The pulmonary veins on the right are visualized, with the intent to inspect the left pulmonary veins once on bypass. As indicated, in the case of a sinus venosus defect the anomalous pulmonary veins should be identified at this point.
Cannulation is commenced by placing purse strings in the superior and inferior venae cavae as well as the aorta. If there are anomalous pulmonary veins in association with a sinus venosus defect, the superior vena caval cannulation site should be at the junction of the SVC and innominate vein in order to be well above the entrance of the anomalous veins. The patient is cannulated and cardiopulmonary bypass instituted. Normothermia is used in most cases of simple ASD. Mild levels of hypothermia (32 to 34°C) may be used when a longer cross-clamp time is anticipated (e.g., sinus venosus ASD with partial veins). Inspection of the heart is then completed. Specifically, the position of the left pulmonary veins and the presence of a LSVC can be ascertained by tilting the heart to the right. After inspection, caval tapes are placed around the superior and inferior venae cavae and a cardioplegia needle is inserted into the aortic root. The aortic cross-clamp is applied, and cardioplegia is delivered to achieve electromechanical silence. During this time, the caval tapes are tightened and the RA opened to collect the coronary venous effluent.
Surgical Repair of Secundum-Type Atrial Septal Defects
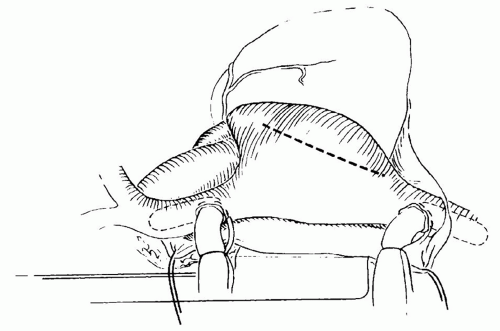
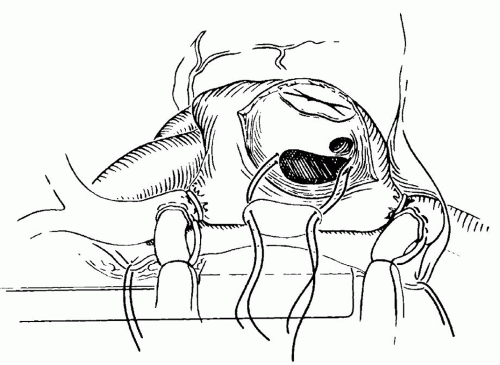
Secundum-type ASDs are approached through a standard right atriotomy (Fig. 79.4). Traction sutures can be placed at the edges of the atriotomy to facilitate exposure. The ASD is identified at its position just beneath the superior limbus. A few moments should be taken to identify the position of the coronary sinus and its relationship to the tricuspid valve. These two structures along with the tendon of Todaro form the triangle of Koch. The AV node should lie at the superior apex of this triangle and may not be too far from the medial border of the ASD. A right-angled clamp can be passed through the ASD into the pulmonary veins to confirm their position. Finally, the position of the inferior vena cava (IVC) cannula should be inspected along with its relationship to the inferior border of the ASD and the Eustachian valve. Misinterpretation of this anatomy can result in inadvertent baffling of the inferior vena caval flow across the ASD into the LA.
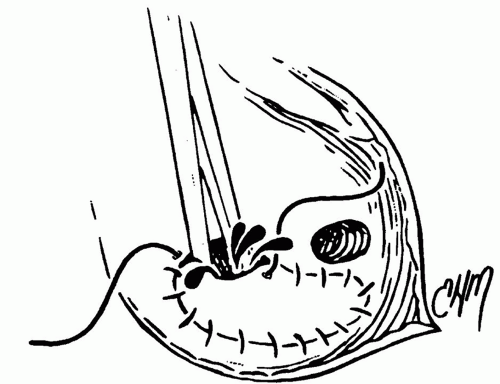
After this checklist of anatomic landmarks is completed, repair of the defect is undertaken. The majority of secundum ASDs in young children can be closed primarily, whereas in teenagers and adults the defects are usually large and the tissues less compliant, so that a patch repair is almost always indicated. Our preference has been to use autologous pericardium, although synthetic patches can also be used. Pericardial patch repair of an ASD is straightforward, and the technique described here is one of the several possible variations. Two separate sutures of 5-0 or 6-0 nonabsorbable vascular suture are placed at the superior and inferior ends of the defect. Each of these sutures is passed through the pericardium at a distance slightly exceeding the length of the ASD (Fig. 79.5). The pericardial patch is cut and lowered into position with the smooth side toward the LA. Each of the sutures is tied, and then each arm of the double-armed suture is run 90 degrees to meet its opposite suture. Just before completing this process, the left side of the heart is filled with blood by ventilating the patient to increase pulmonary venous return and thereby expressing any air remaining in the LA (Fig. 79.6). The patient is then positioned in a steeply head-down position and
the cross-clamp removed with the aortic root on suction. Rewarming is performed while the right atriotomy is closed with a double running 5-0 or 6-0 nonabsorbable vascular suture. The patient is weaned from cardiopulmonary bypass once rewarming is completed.
the cross-clamp removed with the aortic root on suction. Rewarming is performed while the right atriotomy is closed with a double running 5-0 or 6-0 nonabsorbable vascular suture. The patient is weaned from cardiopulmonary bypass once rewarming is completed.
Amplatzer Repair of Secundum-Type Atrial Septal Defects
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree