Arch Aneurysms
Joseph S. Coselli
Susan M. Trocciola
Kim I. de la Cruz
Scott A. LeMaire
INTRODUCTION
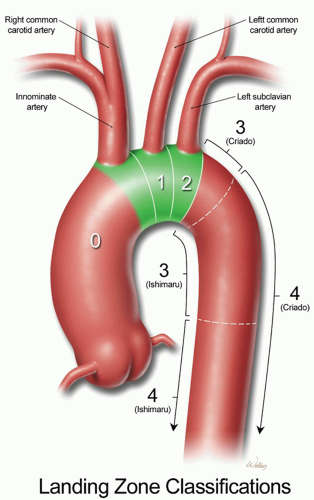
The aorta is divided into two major segments: the proximal aorta and the distal aorta. The proximal aorta includes the aortic root, ascending aorta, and transverse aortic arch. The transverse aortic arch is anatomically defined as the segment of aorta that includes the origins of the brachiocephalic vessels: the innominate artery, left common carotid artery (LCCA), and left subclavian artery (LSCA) (Fig. 61.1). The descending thoracic aorta starts distal to the origin of the LSCA and continues to the diaphragmatic hiatus, where it joins the abdominal aorta. Criado and colleagues, as well as Ishimaru and associates, have each separately mapped the arch into five anatomic zones for endovascular procedures (Fig. 61.1). This schema has mostly been used to describe proximal landing zones for disease that involves the descending thoracic aorta, but it is becoming increasingly valuable in describing combined open and endovascular (hybrid) approaches to aortic arch repair.
Aortic aneurysm can occur anywhere along the aorta and is defined as a permanent, localized dilation that is at least 50% greater than the normal diameter at that anatomic level. Most arch aneurysms are contiguous with an aneurysm involving the adjacent ascending or descending thoracic aorta. Isolated arch aneurysms are less common and generally appear as localized, saccular outpouchings.
Aneurysms of the aortic arch can be caused by a variety of diseases but most commonly result from medial degenerative disease and chronic aortic dissection. Additional causative factors include genetically triggered disorders (such as Marfan, Ehlers-Danlos, and Loeys-Dietz syndromes), infection, trauma, and aortopathy related to having a bicuspid aortic valve.
Surgical repair of the aortic arch remains one of the most difficult challenges in cardiothoracic surgery. This is largely because one must interrupt the natural flow of blood to both the brain and downstream organs. The first successful graft repair of a transverse aortic arch aneurysm was reported in 1957 by DeBakey, Crawford, Cooley, and Morris, who used an early form of antegrade cerebral perfusion (ACP).
For many years, aortic arch surgery was associated with high mortality and neurologic morbidity. Recently, dramatic advances in aortic arch surgery have improved outcomes. These advances include new or refined strategies for cerebral protection and arterial perfusion, revised reattachment strategies for the brachiocephalic vessels, and new options for treating extensive aortic disease. In addition, endovascular techniques that have recently revolutionized distal aortic repair are increasingly being applied to the aortic arch.
DIAGNOSIS AND PREOPERATIVE ASSESSMENT
Although most aneurysms are asymptomatic and are incidentally discovered through imaging studies performed for other reasons, some patients with aortic arch aneurysm present with symptoms, which are often related to the aneurysm’s proximity to nearby structures. For example, patients may present with hoarseness because of stretching of the left recurrent laryngeal nerve. Patients may have dysphagia from esophageal compression, respiratory symptoms due to airway compression, or edema of the upper body from superior vena caval compression. Other problems that can arise from aneurysms involving the aortic arch and the adjacent segments include thromboembolic events such as stroke, and aortic valve regurgitation from associated root enlargement. Chest wall compression can cause chronic dull or aching retrosternal or mid-scapular pain. New severe pain usually indicates aneurysm rupture or acute dissection. Arch aneurysms can rupture into the pleural cavity (usually on the left), mediastinum, esophagus, or tracheobronchial tree.
Dissection involving the arch can cause signs and symptoms of cerebral malperfusion, including syncope and neurologic deficits.
Whereas a chest radiogram may reveal a dilated aorta, definitive evaluation of an aortic arch aneurysm generally requires computed tomography or magnetic resonance imaging. These modalities can image the entire aorta and elucidate its relationship to surrounding structures and brachiocephalic vessels. Echocardiography is generally not useful in evaluating the aortic arch because of acoustic shadowing from the trachea. However, many patients with arch aneurysms have associated ascending aortic, valvular, or cardiac disease; therefore, echocardiography is useful for both planning the operation and assessing risk. Similarly, angiography is generally not used on a routine basis, but it is usually performed in patients who are already undergoing left heart catheterization to evaluate coronary artery disease.
Because there is a well-known association between aneurysmal aortic disease and carotid artery stenosis, it is useful to perform a screening ultrasound of the carotid arteries on patients with arch aneurysms. The risk of stroke is significant during aortic arch repair, and cannulation strategies may need to be revised in an individual patient on the basis of preoperative findings. Additional investigation may include pulmonary function testing, cardiac stress testing, nuclear imaging, and coronary catheterization.
 INDICATIONS
INDICATIONSAortic aneurysms are repaired in order to prevent dissection, rupture, and death. Examining the natural history of aortic aneurysms has helped clarify the risk of these catastrophic complications. Elefteriades and his group at Yale found that aortic diameter is a strong predictor of rupture or dissection, especially when a “hinge point” is reached—a diameter of 6.0 cm in the
ascending aorta or 7.0 cm in the descending aorta. Thus, the investigators recommended replacing the ascending aorta at a diameter of 5.5 cm and the descending aorta at 6.5 cm (5.0 and 6.0 cm, respectively, for patients with Marfan syndrome) or if an aneurysm of any size becomes symptomatic.
ascending aorta or 7.0 cm in the descending aorta. Thus, the investigators recommended replacing the ascending aorta at a diameter of 5.5 cm and the descending aorta at 6.5 cm (5.0 and 6.0 cm, respectively, for patients with Marfan syndrome) or if an aneurysm of any size becomes symptomatic.
Current guidelines make several scenario-based Class IIa recommendations for the repair of aortic arch aneurysms, with the caveat that the approach to repair is generally dictated by adjacent disease of the ascending or descending aorta. For isolated arch aneurysms in low-risk, asymptomatic patients, it is reasonable to replace the arch when the aortic diameter exceeds 5.5 cm or is growing more than 0.5 cm per year. Saccular aneurysms tend to grow more rapidly than fusiform aneurysms and often warrant earlier intervention. For aneurysms limited to the ascending aorta and proximal arch, it is appropriate to perform a partial arch replacement along with the ascending aortic repair. Replacing the entire arch is reasonable in cases of chronic dissection and aneurysms that extend into the proximal descending thoracic aorta. Again, repair is indicated for symptomatic patients regardless of aortic diameter unless the patient has a limited life expectancy. Although the scenario of asymptomatic aortic arch aneurysm in patients with triggered disorders is not specifically addressed in these guidelines, patients with asymptomatic ascending aortic aneurysm in this circumstance may undergo elective repair at an aortic diameter of 4.0 to 5.0 cm, depending on the particular disorder.
PERFUSION AND CEREBRAL PROTECTION
There are several different surgical options for managing aneurysms that involve the transverse aortic arch. The surgical approach depends on the extent of involvement and the need for cardiac and cerebral protection. Repairs of the ascending aorta and arch are performed through a sternotomy with cardiopulmonary bypass (CPB), whereas aneurysms that involve the distal arch and descending/thoracoabdominal aorta are performed through a thoracotomy or thoracoabdominal incision.
Most aortic arch repairs necessitate a temporary period of systemic circulatory arrest combined with varying degrees of hypothermia to protect the brain and other vital organs. Over the last several years, advances in cerebral protection strategies and surgical techniques have been shown to improve outcomes in open arch repair; therefore, we have gradually incorporated these changes into our practice. Changes include the increasing use of ACP rather than retrograde cerebral perfusion (RCP), the use of the axillary or innominate artery instead of the femoral artery as a cannulation site for inflow, and refined hypothermic targets—mild 28.1 to 34°C) or moderate hypothermia (20.1 to 28°C)—rather than deep (14.1 to 20°C) or profound hypothermia (≤14°C).
The well-established role for hypothermia in cerebral protection stems from the fact that the cellular metabolic activity of the brain slows with cooling, reducing cerebral oxygen demand. However, the use of profound hypothermic circulatory arrest (HCA) during arch repair has important limitations; increasing rates of stroke and death have been associated with longer HCA times, especially those longer than 30 minutes. Nevertheless, for selected limited repairs of the aortic arch (i.e., those with an anticipated circulatory arrest time of <30 minutes), the use of HCA alone is considered a reasonable strategy for providing adequate brain
protection. Because many aortic arch repairs are quite complex and require longer periods of HCA, two cerebral perfusion strategies—RCP and ACP—were developed to supplement HCA by delivering cold, oxygenated blood to the brain for the purpose of reducing the risk associated with repair. RCP involves directing blood from the CPB circuit into the head through the snared superior vena cava cannula. Although this approach still has some proponents, recent evidence suggests that RCP is less beneficial than ACP, and today, the use of RCP in aortic arch surgery is generally in decline.
protection. Because many aortic arch repairs are quite complex and require longer periods of HCA, two cerebral perfusion strategies—RCP and ACP—were developed to supplement HCA by delivering cold, oxygenated blood to the brain for the purpose of reducing the risk associated with repair. RCP involves directing blood from the CPB circuit into the head through the snared superior vena cava cannula. Although this approach still has some proponents, recent evidence suggests that RCP is less beneficial than ACP, and today, the use of RCP in aortic arch surgery is generally in decline.
Unlike RCP, ACP provides cerebral flow via the brachiocephalic arteries. Early on, the brachiocephalic vessels were cannulated directly, but this approach was soon abandoned because of the high rate of stroke associated with it. Renewed interest in ACP developed after Japanese surgeons showed improved outcomes with the use of flexible balloon-tipped catheters to deliver ACP during arch repair. Contemporary ACP techniques commonly involve cannulating either the right axillary artery (Fig. 61.2) or the innominate artery and subsequent connection to the arterial limb of the bypass circuit. This procedure carries an inherent risk, although small, of brachial plexus and vascular injury. Once systemic circulatory arrest is induced, the innominate artery is occluded proximally with either a Rumel tourniquet or a vascular clamp, and bypass flow is reduced to approximately 10 ml/kg/min. This allows either the axillary or the innominate artery cannula to deliver oxygenated blood to the brain via the right common carotid artery.
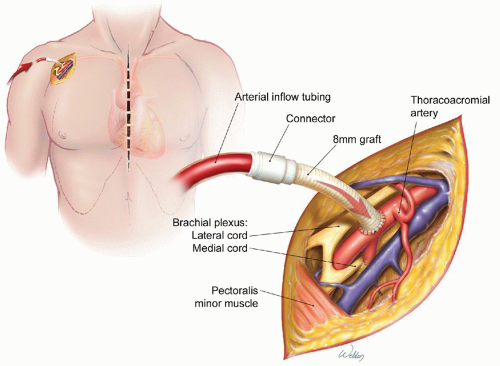 Fig. 61.2. Cannulation of the axillary artery via 8-mm graft for use in delivering cardiopulmonary bypass inflow and antegrade cerebral perfusion. (Image adapted from Gravlee GP, Davis RF, Stammers AH, et al., eds. Cardiopulmonary Bypass: Principles and Practice. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008, Chap. 32, Fig. 1A.) |
When the circle of Willis is intact, unilateral ACP via the right common carotid artery can deliver blood flow to the left side of the brain. Methods to help determine the adequacy of cerebral cross-circulation include preoperative imaging and intraoperative monitoring. Our preferred method of intraoperative monitoring is brain near-infrared spectroscopy (NIRS), which measures cerebral oxygenation; an additional perfusion catheter can be inserted into the LCCA if NIRS monitoring indicates inadequate perfusion. Antegrade cerebral protection via the right axillary artery or innominate artery has become our standard adjunct for cerebral perfusion during HCA and avoids the problems that can develop from cannulating the femoral artery. These problems include plaque embolization, which can result in stroke and other complications; also, in cases of aortic arch dissection, the retrograde pressurization of the false lumen can result in cerebral malperfusion.
Recently, there has been a trend toward using warmer temperatures during HCA with ACP. The primary advantage of mild (28.1 to 34°C) and moderate (20.1 to 28°C) HCA is a reduced risk of the severe and life-threatening coagulopathy that occasionally results from profound hypothermia. Potential drawbacks of warmer temperatures include the increased risk of neurologic deficit, including spinal cord ischemia. Because of the importance of temperature management in patients subjected to HCA, the decision regarding where to measure body temperature is important. The jugular venous bulb provides the best approximation of brain temperature, but few centers use this approach. Nasopharyngeal and esophageal temperatures lag behind the jugular venous bulb temperature but serve as reasonable, practical options. Bladder and rectal temperatures are poor indicators of brain temperature and are generally not reliable during HCA cases.
OPTIONS FOR REPAIR
There are many different options available for repairing aneurysms that extend into the transverse aortic arch; a brief overview of several approaches is presented below. It is important that the surgeon develop a consistent approach that allows the anesthesiologist, perfusionist, and nurses to function as a team with the goal of limiting circulatory arrest and cardiac ischemic times. The guiding principle should be economy of steps (i.e., avoiding unnecessary activities), especially during circulatory arrest. Aneurysms involving only a portion of the transverse aortic arch are usually treated with partial arch repair. For more extensive aneurysms, the entire arch is typically replaced. If the aneurysm extends into the descending thoracic aorta, the aneurysm can be repaired in either one or two stages. Additionally, the arch can be repaired by using open surgical techniques, experimental endovascular techniques, or a combination of both open surgical and endovascular approaches (i.e., hybrid arch procedures).
Partial Arch Repairs
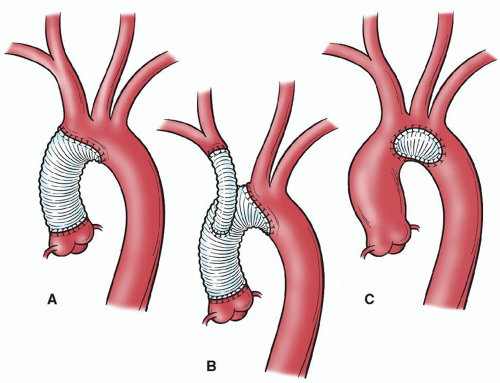
Partial arch repairs, such as patch graft aortoplasty and hemiarch approaches, are less complex than other aortic arch repairs because repair does not directly involve the brachiocephalic vessels. Patch graft aortoplasty can be used to repair saccular aneurysms that originate from the lesser arch curvature and involve <50% of the aortic
circumference. A fusiform ascending aortic aneurysm that extends into only the proximal portion of the arch (in a patient with a normal-sized distal arch) can be repaired with a hemiarch approach by using a beveled graft to replace a portion of the lower curvature of the arch (Fig. 61.3). Of note, for acute dissections that involve the arch (DeBakey I or II), we often use the hemiarch technique.
circumference. A fusiform ascending aortic aneurysm that extends into only the proximal portion of the arch (in a patient with a normal-sized distal arch) can be repaired with a hemiarch approach by using a beveled graft to replace a portion of the lower curvature of the arch (Fig. 61.3). Of note, for acute dissections that involve the arch (DeBakey I or II), we often use the hemiarch technique.
Total Arch Repairs
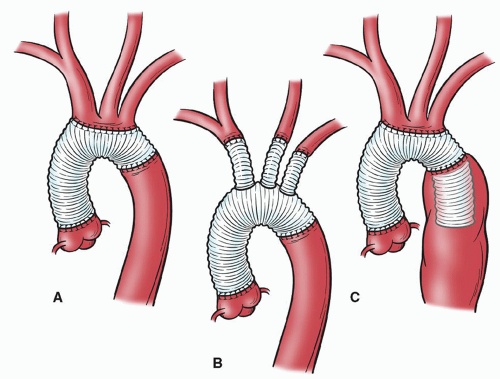
More extensive arch aneurysms often involve total replacement of the arch, with reattachment of the brachiocephalic vessels and a distal anastomosis at the proximal descending thoracic aorta. In the past, we reattached the brachiocephalic vessels to one or more openings made in the graft (“island patch technique”) (Fig. 61.4) or by using smaller, individual grafts to replace each vessel at its origin. There are two disadvantages to the island patch technique. First, this technique can result in difficult-to-control bleeding from the posterolateral aspect of the anastomosis. Second, the aortic tissue left behind can become aneurysmal, necessitating a second operation; this problem is most common in patients with connective tissue disorders.
Y-Graft Techniques
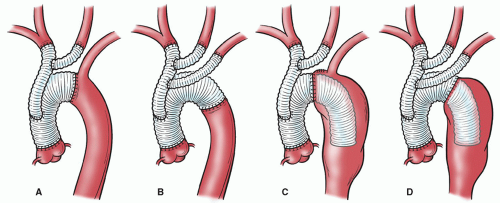
In 2002, Spielvogel and colleagues described the trifurcated graft technique (also called the double Y-graft technique) that they devised to simplify arch reconstruction by essentially “debranching” the aortic arch without strictly following its original anatomic structure. This technique appears to improve outcomes by reducing embolization and minimizing cerebral ischemia. In this technique, single or double Y-branched grafts are created by suturing one or two branch grafts (usually 8 mm in diameter) to a main trunk (usually 12 mm in diameter) at a 45-degree angle; commercially available, prefabricated Y-grafts can also be used. Each brachiocephalic branch vessel is typically sutured end-to-end to separate distal ends of the Y-graft (Fig. 61.5). To expedite repair, debranching can be performed during the cooling process before HCA is achieved. The double Y-graft can then be used to provide ACP while the aortic arch is replaced with a graft. The Y-graft approach is commonly used with the collared elephant trunk graft, and because the brachiocephalic vessels have been debranched, it is possible to move the distal anastomosis forward from its traditional location just distal to the LSCA to a position in line with the origins of the LCCA or innominate artery. These adjustments in technique are thought to promote a hemostatic distal anastomosis.
Elephant Trunk
For extensive aneurysms involving the entire thoracic aorta, repairs are generally performed in two stages, because the
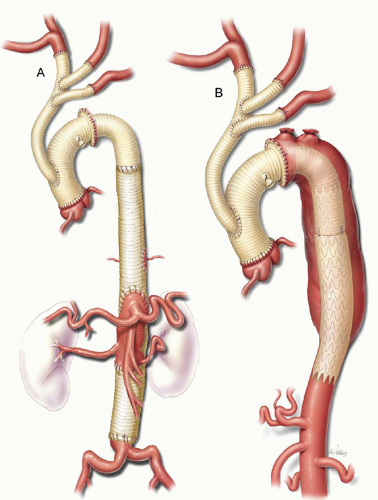
surgical exposure needed to repair the proximal and distal aorta differs. The decision whether to address the proximal or distal aorta first is based on symptoms, aneurysm size, and cardiac disease; the symptomatic segment is addressed first. Borst’s elephant trunk procedure involves repairing the proximal aorta first, then performing a completion procedure to repair the distal portion of the aneurysm. During the first stage of the elephant trunk procedure, the distal anastomosis is constructed and a portion of the graft is left suspended within the proximal descending thoracic aorta (Fig. 61.4C). This “trunk” is then used during subsequent distal aortic reconstruction (Fig. 61.6) and offers many advantages, including easier dissection, shorter durations of aortic clamping, and safer clamping. Avoiding dissection around the distal arch reduces the chance of injury to the pulmonary artery and esophagus, which may be obscured by adhesions. Also, clamp times are usually shorter during the second stage because the proximal anastomosis is graft-to-graft, which makes hemostasis easier to achieve. Finally, because the aorta does not need to be clamped proximal to the LSCA, this technique reduces the risk of stroke from potential embolization of atherosclerotic debris.
surgical exposure needed to repair the proximal and distal aorta differs. The decision whether to address the proximal or distal aorta first is based on symptoms, aneurysm size, and cardiac disease; the symptomatic segment is addressed first. Borst’s elephant trunk procedure involves repairing the proximal aorta first, then performing a completion procedure to repair the distal portion of the aneurysm. During the first stage of the elephant trunk procedure, the distal anastomosis is constructed and a portion of the graft is left suspended within the proximal descending thoracic aorta (Fig. 61.4C). This “trunk” is then used during subsequent distal aortic reconstruction (Fig. 61.6) and offers many advantages, including easier dissection, shorter durations of aortic clamping, and safer clamping. Avoiding dissection around the distal arch reduces the chance of injury to the pulmonary artery and esophagus, which may be obscured by adhesions. Also, clamp times are usually shorter during the second stage because the proximal anastomosis is graft-to-graft, which makes hemostasis easier to achieve. Finally, because the aorta does not need to be clamped proximal to the LSCA, this technique reduces the risk of stroke from potential embolization of atherosclerotic debris.
An important change that improves the elephant trunk approach is the recent introduction of the collared or “skirted” aortic arch graft. The distal anastomosis of the aortic arch for the first stage of the elephant trunk procedure was traditionally done by invaginating the graft within itself,
mimicking an intussusception. One of the problems of this technique was the substantial size discrepancy between the graft and the remaining diseased distal aorta. This size discrepancy could create undue tension on the anastomosis and result in bleeding or pseudoaneurysm formation at a later time. The collared elephant trunk graft facilitates a tension-free distal anastomosis by providing a graft collar that can be easily cut to match the distal aortic diameter; this helps to simplify the repair.
mimicking an intussusception. One of the problems of this technique was the substantial size discrepancy between the graft and the remaining diseased distal aorta. This size discrepancy could create undue tension on the anastomosis and result in bleeding or pseudoaneurysm formation at a later time. The collared elephant trunk graft facilitates a tension-free distal anastomosis by providing a graft collar that can be easily cut to match the distal aortic diameter; this helps to simplify the repair.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree