Fig. 4.1
Aortic valve sclerosis is characteristically a soft, early to mid-systolic ejection murmur in the 2nd right interspace with a normally split S2 without a click
The carotid pulses were normal.
Test Results
LDL 170 mg/dL, TG 330 mg/dL, HDL 35 mg/dL.
An echocardiogram was performed showing aortic valve nodular thickening with an upper normal aortic jet velocity of 1.9 m/s (Fig. 4.2).
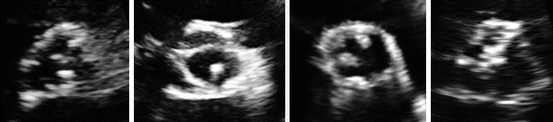
Fig. 4.2
Images showing echocardiographic appearance of aortic valve sclerosis in the parasternal short axis view. Images show from left to right: Mild AVS in its typical location in the noncoronary cusp, mild AVS in an atypical location involving the left coronary leaflet, Moderate aortic valve sclerosis, and severe AVS
Clinical Basics
Definition
AVS has been variably defined across literature. In general, AVS is characterized using echocardiographic imaging by [1, 2]:
Calcification and thickening of a tri-leaflet aortic valve.
Absence of ventricular outflow obstruction.
Aortic jet velocity <2.0 m/s on echocardiogram.
Variant criteria describing AVS have also involved: irregular, non-uniform thickening of portions of the aortic valve leaflets and/or commissures; thickened portions of the aortic valve with calcific appearance; non-restricted or minimally restricted opening of the aortic cusps; and aortic jet velocity <2.5 m/s [2, 3].
Etiology
The etiology of AVS may stem from the fibrosis, thickening, and calcification of aortic cusps. In this way, the precipitating factors of AVS closely resemble those of atherosclerosis [4].
Inflammatory cell infiltrates, oxidized LDL, and osteopontin calcification characterize sclerotic lesions of the aortic valve. These lesions differ from atherosclerotic plaques, however, because AVS lesions have a greater calcium load, uncoupled nitric oxide synthase, and are absent of smooth muscle cell proliferation [2].
Current studies suggest that leaflet and aortic changes are due to an active disease process characterized by sub-endothelial lesions on the aortic side of the leaflets [1]. Theories propose that these changes may be due to calcium spurs on aortic valve cusps or atherosclerotic plaques in the ascending aorta that contribute to turbulent flow [3]. This stands in contrast to previous beliefs that leaflet and aortic changes are due to a non-specific, age-related degenerative process.
Signs and Symptoms
AVS is an asymptomatic condition that is usually only apparent with auscultation. Its sole sign is an ejection flow murmur with a normally split S2 [4].
Click here to listen to an example of an elderly patient with an early mild ejection murmur, classically referred to as the “innocent murmur of the elderly,” unrelated to stenosis of the aortic valve, as described by Dr. W. Proctor Harvey (Video 4.1).
Prevalence
The prevalence of AVS is relative to the age of the patient or population [4].
AVS has been described as a “50–50 Murmur,” meaning that it is present in about 50 % of the population at the age of 50 [4, 5]. From a population perspective, AVS remains an important murmur due to its high prevalence in the aging population.
The prevalence of AVS drops to 30 % of the population at age 50 in more selected patient populations without prevalent cardiovascular disease or left ventricular hypertrophy [3].
Risk factors associated with AVS are similar to those of atherosclerosis and typically include: increasing age, male gender, smoking, HTN, and elevated LDL cholesterol [1, 2]. However, there is some controversy over the association of AVS with gender. Some evidence suggests that AVS may be twice as likely in females [3].
Auscultation Differential Diagnosis
AVS is an ejection murmur.
Ejection murmurs are the result of forward bloodflow through a semilunar (aortic or pulmonary) valve during systole. Ejection murmurs begin at the end of the first heart sound (S1), are crescendo-decrescendo in loudness, end before the second heart sound (S2) on the side of the heart from which the murmur originates, and are louder after long diastoles [4].
The two most common types of ejection murmurs are systolic flow murmurs (murmurs due to causes other than obstruction to flow) and a semilunar valve stenosis ejection murmur [4].
AVS can be classified as a systolic flow murmur, meaning there is no obstruction to flow [4]. There are five key causes of systolic flow murmurs, and the differential diagnosis upon auscultation of a systolic flow murmur should include [4]:
AVS.
Ejection into a dilated artery.
Increased stroke volume or rate of ejection (e.g., anemia, hyperthyroidism).
Normal ‘impulse gradient’ (a murmur only perceivable because of a thin chest or quiet room).
Still’s Murmur.
Clinical Clues to the Detection of the Lesion
AVS should be suspected on auscultation by its characteristic hallmarks [4]:
Soft, early- to mid-systolic ejection murmur in the second right interspace (aortic valve area).
Normally split S2.
Normal volume carotid pulses.
It is important to differentiate AVS from other systolic murmurs, including those due to aortic stenosis, hypertrophic cardiomyopathy, and a bicuspid aortic valve [4].
Similar to AVS, bicuspid aortic valves also produce early ejection murmurs that are loudest in the 2nd right interspace. In contrast to AVS, non-stenotic bicuspid aortic valves are commonly associated with a louder A2 than normal and mild aortic regurgitation [4].
A murmur due to AVS will typically be low grade in terms of loudness, however, grade 3 or greater murmurs can arise from AVS. Such murmurs can be distinguished from aortic stenosis by palpating the carotids. Palpation of the carotids can typically differentiate between the normal rate of rise of the carotids in AVS and the slow rise of the carotids in aortic stenosis [4].
AVS should diminish with maneuvers that diminish ventricular volume and flow (e.g., Valsalva) whereas such murmurs will be accentuated in the setting of hypertrophic cardiomyopathy.
AVS is rarely associated with aortic regurgitation. Thus, if aortic regurgitation is heard, AVS is likely not present [4].
Diagnostic Implications of the Auscultation Features
Currently, there are no objective and established guidelines to classify the severity of AVS according to auscultation features.
Several studies have attempted to characterize AVS severity according to echocardiography features. AVS severity has been graded on a scale from 1–3 based on the amount of aortic leaflet calcification, echocardiography density, aortic valve calcification thickening, aortic leaflet motion, and aortic valve pressure gradient [6, 7] (Fig. 4.2).
Grade 1 or ‘mild’ involves minor calcification of one leaflet and increased echocardiography density.
Grade 2 or ‘moderate’ involves minor calcification of two leaflets or major calcification of one leaflet with valve thickening of calcific deposits >3 mm.
Grade 3 or ‘severe’ involves calcification of all three leaflets or extensive calcification of two leaflets, calcific deposits >3 mm, mildly restricted motion of aortic leaflets, and an aortic valve pressure gradient <16 mm.
It is unknown if auscultation can distinguish between mild, moderate, and severe aortic sclerosis as defined by imaging. However, as noted below, an auscultation diagnosis of AVS may increase the need for surveillance for progression to aortic stenosis.
AVS is associated with both subclinical atherosclerosis and coronary artery disease.
Among a Japanese study of young to middle age individuals, carotid intima media thickness was greater in those with AVS, which persisted after adjustment for cardiovascular risk factors (Fig. 4.3) [8].
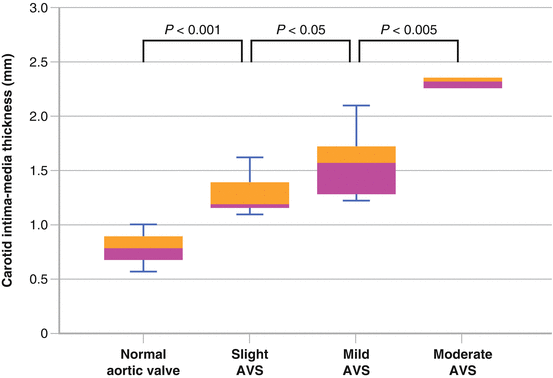
Fig. 4.3
Among a Japanese study of 252 health volunteers aged between 25 and 65 years old, echocardiography and carotid ultrasonography showed that carotid intima media thickness was significantly greater in those with aortic valve sclerosis, which persisted after adjustment for cardiovascular risk factors (Used with permission from Yamaura et al. [8])
Particularly in younger individuals (<65 years of age), AVS suggests increased odds of coronary artery disease (threefold) (Fig. 4.4).
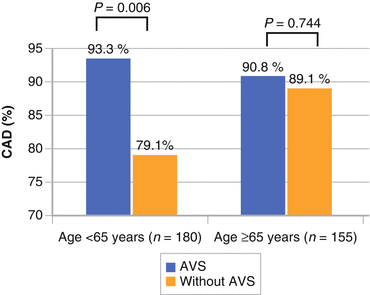
Fig. 4.4
Study of 367 Chinese men and women showing an odds ratio of 3.18 for presence of significant coronary artery disease with aortic valve sclerosis is present (Used with permission from Hsu et al. [22])
Prognostic Implications of the Auscultation Features
AVS is considered a potential ‘precursor lesion’ to aortic stenosis. Patients with AVS are at higher risk for developing aortic stenosis with left ventricular outflow obstruction [1–3, 9, 10].
Calcification, fibrosis, and leaflet stiffness in AVS can increase progressively. This can lead to a reduced systolic opening and an increase in forward velocity that causes hemodynamic abnormality [1–3, 9, 10].
In one prospective trial of 2,131 patients with AVS followed over 7 years, 16 % of patients developed aortic stenosis [11].< div class='tao-gold-member'>Only gold members can continue reading. Log In or Register to continue

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


