Aortic Dissection
Nimesh D. Desai
Joseph E. Bavaria
INTRODUCTION
Thoracic aortic dissection is often a catastrophic disease with an incidence estimated to range from 2.9 to 3.5 per 100,000 person-years in the United States. Appropriate treatment is best managed by a team approach, as the aortic dissection process may affect any part of the circulation leading to potential organ malperfusion of the heart, the brain, the spinal cord, the gastrointestinal tract, the kidneys, and the extremities. A more focused understanding of the causes of death and morbidity of aortic dissection has allowed the development of operations specifically targeted to improve clinical outcomes. Further refinements in management such as the use of the operating room as the diagnostic suite have also contributed to improved survival.
ETIOLOGY
Aortic dissection develops from a tear within the intima of the aortic wall. Blood flows across this “entry point” into a weakened media, splitting the medial layer along the direction of flow, thus creating a new “false” channel, within the aortic media. This new channel progresses downstream and significant pressure/mechanical stress is exerted by the advancing column of blood on the aortic branches encountered in its path. An individual branch will either tear, leading to a communication from the false lumen into the original, “true” lumen of the aorta, or close off causing “malperfusion” of the organ supplied by the given arterial branch. The multiple torn branches down the path of the false lumen become known as reentry points or “fenestrations.” The false lumen, thus, does not usually become a blind pouch with the potential for thrombosis but is kept patent by the many exit and reentry points of variable sizes. If the reentry points are, in aggregate, small or restrictive, a pressure gradient will develop between the false lumen and the true lumen. This pressure differential will cause the false lumen to expand in an amount commensurate to the gradient, causing the true lumen to contract. A new equilibrium is eventually reached with equalization of pressure within the two lumens, typically leaving a small true lumen and a larger false channel. This final equilibrium point is reached within minutes to hours from the time of the original tear, such that the dissection is established and relatively stable by the time the patient reaches medical attention, with a false lumen extending from the entry site most often all the way down to the aortic bifurcation.
Although the final initiating event in an aortic dissection is an intimal disruption, the disease is not an intimal disease. Endothelial cells and their basement membrane do not possess significant intrinsic tensile strength, and their disruption is due to a lack of appropriate mechanical support from the medial layer. The media can be abnormal secondary to a genetic structural abnormality as in Marfan syndrome, where abnormal fibrillin causes loss of elasticity in the medial layer. Marfan syndrome is an autosomal-dominant syndrome with complete penetrance with an overall incidence of 1 per 3,000 to 10,000 live births. It is caused by alterations in the gene coding the aortic wall protein fibrillin-1, leading to elastin derangement. Increased TGF-β activity that negatively affects smooth muscle development and the extracellular matrix has also been implicated. More often other less clearly identified genetic conditions can predispose to abnormalities in the media which lead to dissection. These less well-defined heterogeneous abnormalities are often grouped in the category of annuloaortic ectasia where different abnormalities in the media lead to the common end pathway of dilatation of the aortic diameter with thinning of the aortic wall. Atherosclerotic disease will also damage the media in a variable pattern. Wall weakness in atherosclerotic disease is more often combined with sustained high blood pressure leading to increased stress on the abnormal aortic wall. Within this hypertensive background, a paroxysm of increased arterial blood pressure will incite the initial tear.
CLASSIFICATION
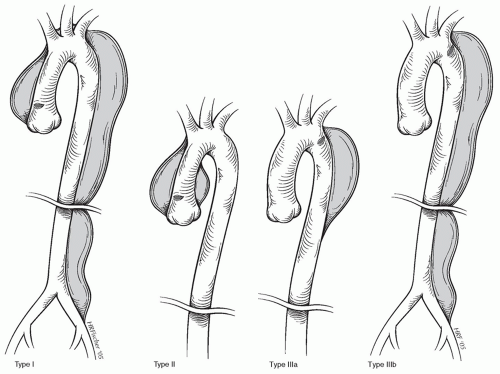
Anatomic classifications of aortic dissection are based on the location of the initial tear and are important in terms of treatment modality and prognosis (Fig. 58.1). The first classification scheme was designed by DeBakey, who classified dissections into the following:
Type I. The process involves the ascending aorta, the aortic arch, and the descending thoracic and often the abdominal aorta.
Type II. The dissection involves the ascending aorta, but stops at the level of the aortic arch, and does not involve the aorta beyond the subclavian artery takeoff.
Type III. The dissection starts distal to the subclavian artery and extends to the entire thoracic aorta (IIIa) or the thoracic and abdominal aorta (IIIb).
The following Stanford classification has became more commonly used as it readily translates to clinical decision-making:
Type A. The dissection involves the ascending aorta, regardless of tear site, and will most often extend into the descending thoracic as well as abdominal aorta. The subsets of DeBakey types I and II are included in this class.
Type B. The dissection starts beyond the aortic arch usually at the subclavian artery and progresses distal, thus not involving the ascending aorta or the aortic arch. The subsets of DeBakey type IIIa and IIIb are included in this class.
The power and popularity of the Stanford classification is due primarily to the very different natural history of the two types of dissection and the requirement of very different surgical approaches to successfully treat them. A type A dissection
carries a high risk of early mortality and morbidity if not treated surgically. A type B dissection has a much lower early mortality, with early surgical treatment providing no clear advantage or even a higher mortality over observation alone except in cases of rupture or severe malperfusion. If the prognosis of type A dissection is so ominous early in the process, this represents a surgical emergency. Type B dissection is a much lower risk aortic process with a relatively low risk of rupture. The primary acute problems caused by type B dissections are malperfusion syndromes of those organs in the path of the descending thoracic or abdominal aorta or the iliac arteries.
carries a high risk of early mortality and morbidity if not treated surgically. A type B dissection has a much lower early mortality, with early surgical treatment providing no clear advantage or even a higher mortality over observation alone except in cases of rupture or severe malperfusion. If the prognosis of type A dissection is so ominous early in the process, this represents a surgical emergency. Type B dissection is a much lower risk aortic process with a relatively low risk of rupture. The primary acute problems caused by type B dissections are malperfusion syndromes of those organs in the path of the descending thoracic or abdominal aorta or the iliac arteries.
CLINICAL PRESENTATION AND DIAGNOSTIC EVALUATION
The aortic media have a high density of nerve endings, so when medial disruption takes place, the acute onset of sharp pain is a nearly uniform presenting symptom, occurring in over 80% of patients. Atherosclerotic degeneration of the media may result in less acute and sharp pain during the dissection progression. In an acute type A dissection, the pain is classically described by the acute onset of sharp, severe chest pain which then migrates to the upper back and is often described as extending/progressing down to the lower back and even the groins depending on the dynamics of growth of the false lumen. Once the false lumen is fully established and has stabilized, the acute pain may change into a more persistent and dull pain, which is often not as easy to localize and may involve the chest and back in variable patterns with additional elements of nausea, abdominal pain, diaphoresis, and shortness of breath. The presence of new focal neurologic deficit suggests aortic arch involvement. Flank pain may also be described in patients who are suffering an acute malperfusion of one of the kidneys. Signs and symptoms of venous hypertension suggest that pericardial tamponade are usually accompanied by poorly perfused extremities, sweaty and clammy appearance, and presyncopal symptoms, consistent with a low cardiac output state. Additional presenting symptoms can be the sudden onset of a cold and pulseless extremity, most commonly the right lower extremity. A right upper extremity vascular impairment is often associated with a neurologic event, especially in a patient with an incomplete circle of Willis. These patients can have a catastrophic hemispheric infarct with a dense contralateral hemiplegia. Rarely, paraplegia can be a presenting symptom. This may occur in cases where a false lumen supplying blood to the spinal cord thromboses or the spinal blood supply is sheared off by an intramural hematoma. Acute paralysis usually associated with signs of spinal shock, which, compounded to other cardiovascular effects of the acute dissection, can
further complicate the early assessment of the patient. Similar paralysis symptoms may occur in the setting of total occlusion of the thoracic or abdominal aorta with no blood flow to the extremities, although this is also associated with pulselessness. Abdominal pain can occur in either type A or type B dissection, and it is often associated with stenosis or occlusion of either the celiac or the superior mesenteric arteries.
further complicate the early assessment of the patient. Similar paralysis symptoms may occur in the setting of total occlusion of the thoracic or abdominal aorta with no blood flow to the extremities, although this is also associated with pulselessness. Abdominal pain can occur in either type A or type B dissection, and it is often associated with stenosis or occlusion of either the celiac or the superior mesenteric arteries.
In a relatively young patient without significant risk factors for atherosclerotic disease, the index of suspicion for aortic dissection should be very high when any of these symptoms are described. Screening tests should include a chest X-ray and an electrocardiogram (ECG). ECG is often nondiagnostic, and occasionally it may show inferior wall changes suggestive of right coronary artery compromise by the dissection. Proximal dissection extension into the left main coronary artery causing occlusion is typically a fatal event, and such patients rarely survive long enough to reach medical care. Chest X-ray is a very nonspecific exam and it may show a dilated mediastinum or a large cardiac silhouette possibly due to an acute pericardial effusion.
Patients with chest or back pain that is not keeping with typical anginal symptoms, particularly in the setting of an abnormal chest X-ray, should undergo emergent contrast computed tomographic (CT) angiography. In most emergency departments today, a CT scan with contrast can be obtained within a few minutes of admission. Most modern multidetector CT scans with contrast will make the diagnosis of aortic dissection with exceptional accuracy and often will be the only test required prior to proceeding to definitive treatment. Rarely in a CT scan, particularly when the scan was done to search for a pulmonary embolism and the intravenous contrast is not well timed, the ascending aorta can show artifactual lines that may be interpreted as a type A dissection. These scans represent the few false-positive studies.
Transthoracic echocardiography occasionally is used to make the diagnosis of aortic dissection by visualizing either the ascending aorta or abdominal aorta. A transthoracic echo may be difficult to carry out and poor windows may limit its usefulness. If the aortic root is clearly visualized, a variable degree of aortic valve insufficiency with a dilated root or the suggestion of an intimal flap will suggest the diagnosis of type A dissection. Acute dissection flaps are not always visible with this technique, and transthoracic echo cannot definitively rule out aortic dissection. Pericardial effusion may also be seen.
After a screening transthoracic echo is suggestive but not diagnostic of an aortic dissection, a transesophageal echocardiogram (TEE) can nearly always make an accurate diagnosis of the type of dissection present. While there is no need to delay definitive treatment by adding transesophageal echo to a diagnostic contrast CT scan, in our recent experience it is as accurate as any other diagnostic study and allows rapid evaluation of an unstable patient. This is usually done in the operating room with a full cardiac surgical team available.
Contrast magnetic resonance imaging (MRI) provides excellent images of any dissection; it is not often readily available and it usually requires long acquisition times with the patient not as closely monitored because of the technical requirement of the high magnetic field. It should not be used as a primary modality except when it is more rapidly available than TEE, and the patient has evidence of renal dysfunction, making contrast CT angiography less desirable. In cases where the index of suspicion for acute aortic dissection is high, even in cases of renal dysfunction, CT angiography with contrast provides the most rapid and definitive diagnosis and should be used to avoid unnecessary delays in treatment. MRI can be extremely valuable in following a type B dissection after the initial diagnosis or in evaluating any chronic dissection.
Diagnostic catheter-based aortography has become an uncommon primary diagnostic evaluation. Typically, diagnosis is made by aortography only when the patient presented with an acute coronary syndrome with coronary malperfusion or diagnosed at the time of another cardiovascular intervention causing iatrogenic aortic dissection. Taking a patient with acute aortic dissection to the cardiac catheterization lab carries a very high mortality. Suspecting coronary disease leads to the use of platelet-inhibiting agents, anticoagulants, and occasionally fibrinolytics, all of which can transform a contained rupture into a free disruption or can increase the diffuse blood extravasation occasionally noted in a dissected, extremely thin aorta. Catheter manipulations performed can cause dangerous direct damage to the freshly dissected aorta. When obtained, aortography will only show the presence of a dissection flap and does not provide the anatomic detail a standard CT scan with contrast, a TEE or an MRI can provide today.
The question occasionally arises over the value of elective coronary evaluation after making the diagnosis of type A dissection. Retrospective reviews suggest that the incidence of coronary disease in this population is sufficiently low that more patients are placed at risk by the delay and technical risks of a cardiac catheter than are saved by the discovery of occult coronary disease. The only exception may be in patients who have undergone previous coronary bypass surgery, where the knowledge of coronary disease, graft position, and patency can contribute to a safer operation. When the technology of intraoperative coronary angiogram is available in a dedicated hybrid operating room suite, that may become an option for patients after completion of the proximal repair.
Once the diagnosis of a type A dissection is suggested by any screening test, the patient should not be delayed in the emergency department by further testing but should be transferred to an appropriate institution that can proceed with expeditious surgical repair. Over the last several years, our policy has been to transfer any patient with a documented or highly suspected type A dissection immediately to the operating room to minimize any delay. As soon as the patient arrives to the operation room, if sufficient suspicion or good studies are available, the patient is placed under general anesthesia and a TEE is obtained. If dissection is demonstrated by transesophageal echo in the ascending aorta, the patient undergoes emergency surgery. With this policy, 5% to 10% of patients admitted directly to the operating room are found to have a negative transesophageal echo and are then transferred to the intensive care unit for further evaluation. Most commonly, motion artifact in younger patients, type B dissections with some degree of arch involvement but no flap in the ascending aorta, or intramural hematomas are present in these patients.
ACUTE TYPE A DISSECTION
Patients with an acute type A dissection have a risk of death traditionally estimated at 2% per hour in the first 48 hours from their presentation. More recent data suggest that the early mortality may be as low as 60% over the first 2 to 5 days in patients without a concomitant root aneurysm and with aggressive use of antihypertensive medications, especially beta blockers.
Either way, medical therapy generally has no role in acute type A dissection except to allow a safer transfer to a center with experience in complex aortic repair or in inoperable patients. Historically, type A dissection surgery was focused on placing a short segment of Dacron graft in the ascending aorta in the hope of eliminating the entry tear and obliterating the false lumen both proximally into the aortic root and distally into the arch and beyond. This was frequently done by sewing the Dacron graft proximally to the aorta just proximal to the tear with no stabilization of the aortic root or valve and sewing distally to clamped aorta and not utilizing an open arch anastomosis. That principle often led to recurrent malperfusion proximally (myocardial infarctions and recurrent aortic insufficiency) and distally (stroke, organ, limb malperfusion) as well as a moderate distal early rupture rate. Furthermore, a high incidence of long-term failure was seen with aneurysmal degeneration of both the root and the distal ascending aorta and aortic arch.
Either way, medical therapy generally has no role in acute type A dissection except to allow a safer transfer to a center with experience in complex aortic repair or in inoperable patients. Historically, type A dissection surgery was focused on placing a short segment of Dacron graft in the ascending aorta in the hope of eliminating the entry tear and obliterating the false lumen both proximally into the aortic root and distally into the arch and beyond. This was frequently done by sewing the Dacron graft proximally to the aorta just proximal to the tear with no stabilization of the aortic root or valve and sewing distally to clamped aorta and not utilizing an open arch anastomosis. That principle often led to recurrent malperfusion proximally (myocardial infarctions and recurrent aortic insufficiency) and distally (stroke, organ, limb malperfusion) as well as a moderate distal early rupture rate. Furthermore, a high incidence of long-term failure was seen with aneurysmal degeneration of both the root and the distal ascending aorta and aortic arch.
As better understanding of the etiology for early complications and death was gained, a better operation could be designed to address those factors. There are four primary causes of early death from type A dissection, and the modern approach to surgical correction involves an operation addressing these four problems appropriately and directly.
Aortic rupture. The ascending aorta is subjected to more stress than any other portion of the aorta. It is the site of the majority of medial degeneration and thus intimal tears. Once dissection has occurred, the ascending aorta is at very high risk of early rupture. This risk is magnified when the aortic root and ascending aorta are dilated as the tension exerted on the remaining media and adventitia increases according to the Laplace law by the square of the diameter. To prevent this lethal complication, the ascending aorta needs to be completely replaced.
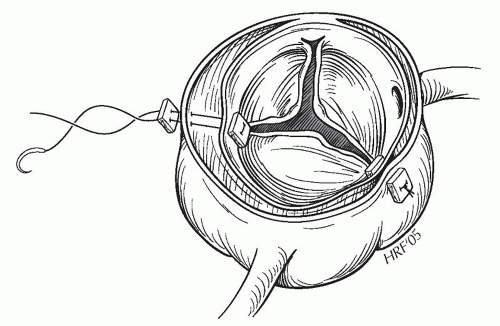
Congestive heart failure due to acute onset of aortic valve insufficiency. Aortic valve support is lost due to prolapse of the intimal layer at the level of one or more commissures. The most commonly affected is the noncoronary to right coronary sinus commissure, followed by the noncoronary to left coronary sinus and least common is the right coronary to left coronary commissure. To eliminate aortic valve regurgitation, the operation should include either a repair or a replacement strategy as necessary. The most common repair technique consists of aortic valve resuspension to reestablish normal commissural support (Fig. 58.2). Furthermore, the sinotubular junction may be dilated and may need to be brought back to a geometrically normal size. The ascending aortic graft is sized to best return the sinotubular dimension to its normal state.
Acute myocardial infarction due to malperfusion of the coronary arteries. Very few patients with left main coronary compromise survive to reach medical attention. Right coronary malperfusion is much more common. At surgery, the root needs to be repaired definitively to treat ongoing coronary stenosis/occlusion or to prevent future coronary compromise or late root dilatation. The incidence of aortic root dissection is quite high, especially in the noncoronary sinus where the great majority of aortic root will often be dissected down to the annulus. The second most commonly dissected sinus is the right coronary sinus with often some element of coronary malperfusion. Occasionally, the left coronary sinus of Valsalva will be also dissected and while in the past that was considered to be an indication for root replacement, as long as the intima within the root is intact and the valve leaflets are otherwise normal, dissection of the left coronary sinus can be also managed by repair techniques. Our preferred technique consists of reinforcing any dissected component of the root with an appropriately fashioned Teflon-felt sheet, placed between the intima and the adventitia becoming essentially a neomedia (Fig. 58.3
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree