Alternative and Backup Methods to Improve Interpretation of Concerning FHR Patterns
The majority of fetal heart rate (FHR) patterns seen in labor at some point will evolve into those that are neither clearly indicative of fetal hypoxia (Category III) nor clearly not associated with hypoxia (Category I) and thus fall into Category II. As clarified in the bulletin by the American Congress of Obstetricians and Gynecologists (ACOG), some of these patterns, such as milder degrees of variable decelerations with average variability, are unlikely to be indicative of a deteriorating or concerning status (1). And, while interventions such as oxygen, fluid, and position change may be warranted, these patterns can be watched to be sure they do not deteriorate into one that is more concerning. Others in this category, such as recurrent late decelerations with moderate or reduced variability, are indeed associated with some degree of hypoxia, and the question becomes, is the hypoxia severe enough that the fetus has become or is becoming acidotic as well? As a basic premise, the purpose of electronic fetal monitoring (EFM) is to detect fetal hypoxia and acidosis at a point where, if it cannot be reversed, intervention by cesarean section or operative vaginal delivery can occur before damage to fetal tissues and organs or fetal death occurs. As repeatedly pointed out elsewhere in this text, the limitation of EFM is primarily that many other variables affect the FHR and may mimic changes that occur when there are hypoxia and the resulting acidosis. Thus, in many situations, some alternative or “backup method” for more specifically assessing the degree of hypoxia and/or acidosis is needed.
When EFM was first coming into general use in the United States and still in many countries, particularly in Europe, this question is answered with scalp pH sampling. But in the United States and many other countries, scalp pH sampling has been largely abandoned due to its technical difficulty, its need for frequent repetition, and the problem with distinguishing between relatively innocuous and more common respiratory acidosis and far more serious metabolic acidosis. Several newer methods of assessing fetal status in the face of more concerning Class II patterns have evolved since the introduction of scalp pH sampling including the utility of spontaneous or evoked FHR accelerations, fetal pulse oximetry, fetal electrocardiogram (ECG) waveform analysis (STAN technology), and computer interpretations of FHR pattern. These backup methods, the reasoning behind them, and their strengths and weaknesses will be the subject of this chapter. A few other methods that showed promise for this purpose such as continuous pH monitoring and fetal pO2 monitoring have been tried experimentally but never put into clinical practice and will not be discussed.
FETAL SCALP pH SAMPLING
The physiologic basis of the respiratory changes that occur with substantial and prolonged hypoxia, the conversion to anaerobic metabolism, and the resulting acidosis are discussed in Chapter 2. Ideally, the direct measurement of oxygen levels or pH would answer the question of the severity of hypoxia and/or the presence of acidosis and thus the logic behind fetal scalp pH sampling that came into use shortly after or concurrently with the introduction of EFM. While not a measurement of central or arterial pH as would be ideal, the fetal scalp is usually the only part that can be accessed in labor, and measurement of the pH from capillary blood obtained from the scalp in most situations accurately provides information that can be used to predict the central pH of the fetus at any given point in time.
Equipment Necessary for Fetal Scalp Blood Sampling
Before sampling fetal blood, the proper equipment must be available, and there must also be an assistant to help hold the patient in position, make notations on the monitor, connect the light source to the battery, accept the filled capillary tubes from the physician, and prepare them properly for the laboratory.
A sterile tray (now available with disposable items) should contain the following: (a) four or five 200-µL heparinized capillary tubes, (b) a conical endoscope with light source, (c) a 2-mm blade on a long handle, (d) silicone grease, (e) 10 or 15 sponges, and (f) a long-handled sponge holder. The physician should wear sterile gloves, and the patient should be prepped and draped in a sterile manner.
Technique of Fetal Scalp Blood Sampling
The optimum position of the patient for fetal scalp blood sampling is important. The lithotomy position, with a patient in stirrups and the maternal buttocks extending over the edge of the table, is preferred by most. This can be done in most convertible labor beds, but, if not available, it is easily accomplished in the delivery room. The lateral Sims’ position is also satisfactory, requires less patient movement, and allows the patient to remain in the lateral position. When using the lateral Sims’ position, it is important that the patient be well flexed at the hip with the lower leg extended. The upper leg is held by an assistant with the patient’s buttocks extending well over the edge of the bed to allow the person taking the scalp sample to be positioned below the level of the maternal vagina. With both lithotomy and Sims’ techniques, the most important factor is for the scalp sampler to be able to angle the cone toward the maternal spine.
With the patient in position, the cone (with light source) is inserted into the posterior fornix under direct visualization. Once the cone is inserted past the anterior lip of the cervix, the cone is angled anteriorly into the cervix and the presenting part is visualized. A sponge is used to wipe the scalp surface clean, and then silicone grease is applied to form a nonwettable surface that will allow the fetal scalp blood to form in easily accessible beads. A standard fetal scalp blade with a depth of 2 mm is then used with a quick “stab” to make a clean incision and blood will appear. A 200-µL heparinized capillary tube is then inserted to touch the drop of blood, and keeping the tube angled downward, the blood is allowed to flow by gravity. About one-fourth of a tube of blood without bubbles is needed for a pH, but for complete fetal scalp blood gases (pH, pCO2, and base deficit), the tube should be about three-fourths full. After taking the sample, the capillary tube should be immediately handed to an assistant for proper sealing and mixing with a magnetic “flea.” Pressure with a sponge should be kept on the scalp wound through the next two contractions, and it should then be observed during another contraction to be sure the bleeding has stopped. Sometimes, more pressure is required, and other times (rarely), it may be necessary to put a skin clip on the wound to stop the bleeding. Once fetal scalp blood sampling has been done, continued observation of the patient must be carried out, as even what appears to be “heavy show” during labor may be significant fetal hemorrhage from the scalp puncture site (2).
Indications for Fetal Scalp Blood Sampling
Total agreement on the indications for fetal scalp blood sampling does not exist. Certainly, if an institution cannot provide 24-hour ready access to accurate micro blood gas analysis with a 10- to 15-minute turnaround time, fetal scalp blood sampling should not be used. The ability to implement decisions for rapid operative intervention is also necessary to effectively use this technique.
Given the necessary logistical support, the indications for fetal scalp blood sampling should be limited to patients who are in labor with ruptured membranes and cervical dilatation sufficient to allow introduction of the cone (usually 2 to 3 cm) and with the fetal head at a station that is within 2 cm of the spines. Fetal scalp blood sampling for acid-base studies should be limited to patients who have electronic FHR tracings suggestive of hypoxia or to clarifying a confusing pattern such as absent variability without decelerations or accelerations. The better one understands FHR monitoring, the less necessary fetal scalp blood sampling will be. Examples of situations where fetal scalp sampling may remain a good choice in modern clinical practice include
A confusing FHR pattern is present with elements that suggest fetal hypoxia.
There is a sustained flat FHR, especially at the time of admission, without significant periodic changes.
Uncorrectable late deceleration with moderate or minimal variability is present in a patient for whom vaginal delivery is anticipated within 1 to 2 hours.
Correlation between Fetal Heart Rate Patterns, Fetal pH, and Outcome
A pH of 7.25 or greater indicates the absence of acidosis and the pattern can be safely watched unless it worsens, a value between 7.20 and 7.25 is equivocal and needs to be repeated immediately, and a value of <7.20 is consistent with acidosis and should warrant operative intervention unless delivery is imminent. It should be reemphasized that whenever possible the fetal pCO2 should be simultaneously measured and taken into account. A respiratory acidosis alone is not an indication for delivery. Only if acidosis occurs and appears to be metabolic should a low pH be considered potentially harmful to the fetus.
Early studies on the correlation between FHR patterns and fetal scalp blood pH revealed that there was at least a general correlation (3,4,5,6,7,8,9 and 10). Kubli et al. (11) showed that it was indeed rare to have a fetal scalp blood pH value below 7.20 with an innocuous FHR pattern. However, many patterns of late deceleration and moderate-to-severe variable deceleration were often associated with fetal scalp blood pH values above 7.20. Furthermore, approximately 10% of fetal scalp blood pH samples obtained at the time of delivery were found to be below the values found in the umbilical artery (12).
The correlation between fetal scalp blood pH measurement and neonatal Apgar score increases as the sample is taken closer to the time of birth. With samples taken within 5 minutes of delivery, Hon and Khazin (5) and Modanlou et al. (6) showed that the correlation between low scalp pH and low Apgar scores at both 1 and 5 minutes was very high. However, there appears to be a rather poor correlation between fetal pH and Apgar scores between 7 and 10, with a relatively high incidence of falsely low values. This may be accounted for partially by local factors such as stasis that may make the fetal pH low at the scalp when the central fetal circulation is normal, especially at the time of delivery when caput formation is the greatest.
The correlation between fetal scalp blood pH measurement and neonatal Apgar score increases as the sample is taken closer to the time of birth. With samples taken within 5 minutes of delivery, Hon and Khazin (5) and Modanlou et al. (6) showed that the correlation between low scalp pH and low Apgar scores at both 1 and 5 minutes was very high. However, there appears to be a rather poor correlation between fetal pH and Apgar scores between 7 and 10, with a relatively high incidence of falsely low values. This may be accounted for partially by local factors such as stasis that may make the fetal pH low at the scalp when the central fetal circulation is normal, especially at the time of delivery when caput formation is the greatest.
Therefore, scalp pH appears to be a reliable method of backing up a concerning FHR pattern. The limitations are however substantial primarily from a technical difficulty standpoint and the need to repeat the test every 20 to 30 minutes, and thus, the test is rarely used in the United States. Thus, there is a real need for an alternative method to clarify FHR patterns that are concerning for hypoxia and acidosis (Category II) but not more clear-cut as with Category III patterns.
ACCELERATIONS OF THE FHR AS A SUBSTITUTE FOR FETAL SCALP BLOOD SAMPLING
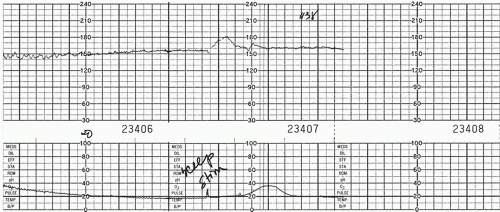
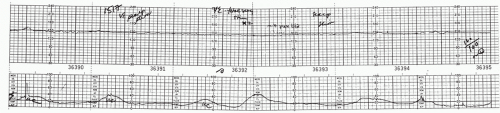
In 1982, Clark et al. (13) reported on 200 patients who had fetal scalp blood sampling and noted that none were acidotic if there was an FHR acceleration associated with the fetal scalp blood sampling. In a subsequent prospective study, they found that no fetus with a fetal scalp blood pH below 7.19 demonstrated acceleration at the time of scalp sampling. (14) In 1986, Rice and Benedetti (15) showed that, in patients with a concerning FHR pattern, 70 of 71 fetuses with acceleration had a pH above 7.20, whereas 7 of 32 with no acceleration had fetal scalp blood pH values below 7.20 (Figs. 9.1 and 9.2).
Smith et al. (16) reported on a similar correlation between fetal acceleration in response to sound stimulation with an artificial larynx (also known as a vibroacoustic stimulator). In their study of fetuses with abnormal FHR patterns, all 30 fetuses that showed one or more accelerations in response to sound had a pH above 7.25, while 18 of 34 that showed no acceleration with sound were acidemic. It has subsequently become clear that accelerations regardless of whether they are occurring spontaneously or elicited by scalp or acoustic stimulation have a similar meaning (i.e., the fetus is not acidemic). The converse however is not necessarily true. The fetus that is not moving will not have accelerations regardless of its acid-base status, and there will be no accelerations present. Thus, the primary place for judging whether the fetus is acidotic or not based on accelerations is in the situation where the remainder of the FHR pattern is concerning (certain types of Category II more likely to be associated with fetal hypoxia). In this setting, accelerations, either spontaneously occurring or elicited by fetal scalp stimulation or acoustic stimulation, indicate that the fetus fetal scalp blood pH at that point in time would unequivocally NOT be acidotic. If the fetus does not respond with acceleration to either of these stimuli, in the setting of an otherwise concerning FHR pattern, it would appear that approximately 50% will show acidosis on a simultaneously obtained fetal scalp blood sample. Because most hospitals with obstetrics units do not have fetal scalp sampling available (17), this approach has become the alternative to fetal scalp sampling. In the vast majority of institutions in the United States, fetal scalp stimulation and acoustic stimulation have virtually replaced fetal scalp blood sampling without an increase in cesarean section rates for nonreassuring fetal status on the EFM recording (18). It is inappropriate to use scalp or vibroacoustic stimulation during a deceleration. During an ongoing prolonged deceleration or bradycardia, first there are no data in the literature to help understand whether the presence or absence of an acceleration has the same meaning. More importantly, even if the acceleration did mean the absence of acidosis at that point, there is no assurance that, in the face of potentially rapidly deteriorating oxygenation, a few minutes later the fetus would not become acidotic. The value of these techniques is when the stimulation occurs during a time when the FHR is at its baseline rate and the acceleration evoked is above the baseline. Also, like fetal scalp sampling, if an acceleration does occur but the pattern remains the same or worsens, an acceleration must be seen every 20 to 30 minutes to again be reassured of the absence of acidosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree