Fig. 2.1
Heart failure staging system (Adapted from Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005 Sep 20;112(12):e154–235)
Importance of Algorithms
In order to define and guide the optimal management of HF patients in varying clinical scenarios, treatment algorithms have become an essential cornerstone of clinical practice. These modalities are valued for their ability to help streamline clinical decision making based on disease severity. However, oversimplification of an algorithm may lead to its inapplicability in complex clinical situations. Therefore, treatment algorithms should be based on current guidelines derived from large randomized controlled clinical trials and individualized based on the assessment of a clinical situation.
In the field of heart failure, there are five main sets of guidelines developed by (1) European Society of Cardiology (ESC 2012), (2) American College of Cardiology/American Heart Association (ACC/AHA 2009), (3) Heart Failure Society of America (HFSA 2010), (4) Canadian Cardiovascular Society (CCS 2012), and (5) International Society of Heart and Lung Transplantation (ISHLT 2007). The algorithm described in Fig. 2.2 is based on these guidelines as well as current randomized controlled trials.
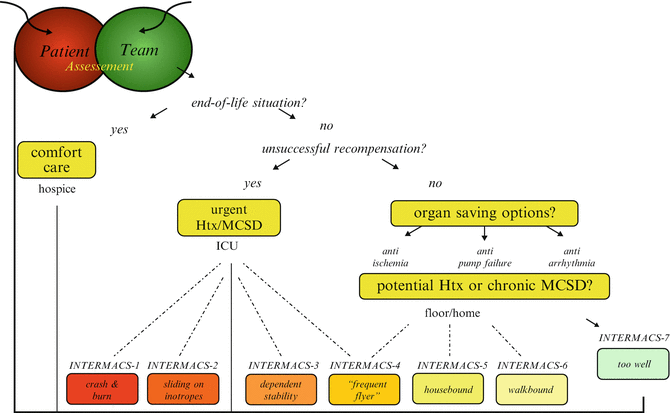

Fig. 2.2
Management algorithm in heart failure (Adapted from Deng MC, Naka Y. Mechanical Circulatory Support Therapy for Advanced Heart Failure. London: Imperial College Press; 2007)
Initial Assessment
The algorithm starts with the encounter between the HF patient and the primary medical team, consisting of cardiologist, general internist, and nurse, who have exhausted all lifestyle and medical options without success. In this setting of acute decompensation and progression towards advanced heart failure, a phase known to be associated with a high risk of death, a referral to a designated cardiac transplantation center for evaluation is undertaken. The initial assessment is not a complete cardiac transplantation evaluation but rather addresses the following main questions:
How severe is the heart failure condition?
Are there reversible causes?
Are there risk factors limiting the overall prognosis?
After the initial assessment, a structured management algorithm (Fig. 2.2) is applied in order to recompensate the patient. If recompensation cannot be achieved, cardiac transplantation evaluation is initiated with the option of mechanical circulatory support device (MCSD) as either bridge to recovery (BTR), transplant (BTT), or destination therapy (DT). At anytime during management, a situation may arise in which the patient may not benefit from any of the modern therapies because of multiorgan failure or other conditions, leading to a patient preference for comfort care facilitating a humane form of death instead of prolongation of suffering [13, 14].
Risk Stratifiers
In order to plan effective treatment strategies and transplant programs, it is important to be able to objectively measure the prognosis of patients. An ideal test needs to be accurate (i.e., have a high specificity and sensitivity), reproducible, safe, and inexpensive.
The 6-min walk test can be performed by almost all patients with chronic heart failure without the need for specialized equipment. This test was first used in heart failure patients by Guyatt and colleagues in 1985 [15] and has subsequently gained widespread acceptance as a measure of exercise capacity in clinical trials and transplant programs. Zugck et al. showed that the walk test provided information on the combined end point of death and/or hospital admission due to worsening heart failure that was similar to peak oxygen uptake in patients with dilated cardiomyopathy [16]. The authors concluded the test correlated closely with peak oxygen uptake (pVO2) and could predict individual pVO2 when determined serially in the same patient. Opasich and colleagues also compared the prognostic role of the 6-min walk test to pVO2 and NYHA functional class. Although the test was found to be able to predict survival in univariate analysis, this was not the case when pVO2 or NYHA class were included in multivariate models, indicating that the walk test is not an independent prognostic indicator [17]. Whether the test is an accurate and independent predictor of prognosis in chronic heart failure, however, is the subject of some debate [18].
Peak Oxygen Uptake
Based on the groundbreaking work of Mancini and coworkers [19], a team at UCLA assessed the role of pVO2 in reevaluation of candidates awaiting heart transplantation. All ambulatory transplant candidates with initial pVO2 ≤14 mL/kg/min were identified. Of 107 such patients listed, 68 survived without early deterioration or transplantation to undergo repeat exercise. In 38 of the 68 patients, pVO2 increased by ≥2 mL/kg/min to a level ≥12 mL/kg/min after 6 ± 5 months, together with an increase in anaerobic threshold, peak oxygen pulse, and exercise heart rate reserve and a decrease in heart rate at rest. Increased pVO2 was accompanied by stable clinical status without congestion in 31 of 38 patients, and these 31 were taken off the active waiting list. At 2 years, actuarial survival rate was 100 %, and survival rate without relisting for transplantation was 85 %. The authors concluded that an algorithm with scheduled reevaluation of exercise capacity and clinical status allowed identification of patients who became “too well” during follow-up. They estimate that 29 % of ambulatory transplant candidates could be removed from the waiting list with excellent early survival despite low pVO2 on initial testing, allowing deferral of transplantation in favor of more compromised candidates [20].
In order to refine risk stratification in ambulatory cardiac transplantation candidates and estimate their survival probability without transplantation and thus the potential benefit from transplantation, the group at the University of Pennsylvania and Columbia University between 1987 and 1995 developed the first independently validated prognostication tool, entailing high-, medium-, and low-risk stratum [21]. The multivariable proportional hazards survival model was developed with the use of data on 80 clinical characteristics from 268 ambulatory patients with advanced heart failure (derivation sample). Invasive and noninvasive models (with and without catheterization-derived data) were constructed. Stratum-specific likelihood ratios were used to develop three prognostic-score risk groups. The noninvasive model performed well, and increased performance was not attained by the addition of catheterization-derived variables.
Prognostic-score risk groups derived from the noninvasive model in the derivation sample effectively stratified the risk of an outcome event in both the derivation and validation samples (1-year event-free survival for derivation and validation samples, respectively: low risk [Heart Failure Survival Score or HFSS 8.10–10.47] 93 % and 88 %; medium risk [HFSS 7.20–8.09] 72 % and 60 %; high risk [HFSS 5.51–7.19] 43 % and 35 %). The authors concluded that selection of candidates for cardiac transplantation may be improved by use of this noninvasive risk-stratification model [21]. The beauty of this score does not reside only in its powerful predictive value but also on its easy bedside implementation by the equation:
![$$\begin{array}{llll}\text{HFSS}=[\left(0.69\times \text{CAD}:\text{YES}=1\text {NO}=0\right)\\ +\left(0.022\times \text{HR}\right)+\left(-0.046\times \text{LVEF}\right)\\ +\left(-0.026\times \text{mBP}\right)\left(0.61\times \text{IVCD}:\text{YES}=1\text{NO}=0\right)\\ +\left(-0.055\times {\text{VO}}_{\text{2}}\right)+\left(-0.047\times \text{Na}\right)]\\ \text{CAD}\text{coronary}\text{artery}\text {disease};\text{HR}\text {heart}\text {rate};\text{LVEF}\\ \text{left} \text{ventricular}\text {ejection}\text {fraction};\text{ mBP}\text {mean}\text {blood}\\ \text{pressure};\text{IVCD}\text {interventricular}\text {conduction}\text {delay};\\ {\text{VO}}_{2}\text{ peak oxygen consumption Na}\text {sodium}.\text{ gathered}\end{array} $$](/wp-content/uploads/2016/09/A312602_1_En_2_Chapter_Equ0002a.gif)
![$$\begin{array}{llll}\text{HFSS}=[\left(0.69\times \text{CAD}:\text{YES}=1\text {NO}=0\right)\\ +\left(0.022\times \text{HR}\right)+\left(-0.046\times \text{LVEF}\right)\\ +\left(-0.026\times \text{mBP}\right)\left(0.61\times \text{IVCD}:\text{YES}=1\text{NO}=0\right)\\ +\left(-0.055\times {\text{VO}}_{\text{2}}\right)+\left(-0.047\times \text{Na}\right)]\\ \text{CAD}\text{coronary}\text{artery}\text {disease};\text{HR}\text {heart}\text {rate};\text{LVEF}\\ \text{left} \text{ventricular}\text {ejection}\text {fraction};\text{ mBP}\text {mean}\text {blood}\\ \text{pressure};\text{IVCD}\text {interventricular}\text {conduction}\text {delay};\\ {\text{VO}}_{2}\text{ peak oxygen consumption Na}\text {sodium}.\text{ gathered}\end{array} $$](/wp-content/uploads/2016/09/A312602_1_En_2_Chapter_Equ0002a.gif)
Event-free survival rates for the medium- and high-risk strata were much worse than would be expected after cardiac transplantation; the low-risk stratum had an event-free survival rate that was better than would be expected with transplantation. Based on this excellent prognostication tool, patients with HFSS low risk would be considered too well for cardiac transplantation [21]. Risk stratification of hospital-bound cardiac transplantation candidates who are inotrope- or left ventricular assist-device-dependent can be improved by inclusion of further parameters [22].
After the introduction of β-blocker therapy and given the large survival benefit conferred by β-blocker therapy, it was unclear whether the HFSS and pVO2 were still valid predictors. The fact that β-blockers considerably improved survival while having an inconsistent effect on pVO2 may explain why pVO2 did not accurately predict outcomes in patients taking β-blockers. Given the better prognosis for patients with heart failure receiving β-blockade and absence of effect on exercise performance, the clinical guideline value for pVO2 has probably decreased to the extent that a pVO2 ≤10 mL/kg/min is a more appropriate target. However, recalibration of the HFSS was not necessary since there were no particular differences in the HFSS pre- or post-β-blocker therapy or its parameters (other than heart rate). The authors conclude that in the β-blocker era, clinicians can continue to rely on the HFSS to accurately predict prognosis in patients with severe heart failure and that pVO2 may have diminished in value [23].
The predictive accuracy of the HFSS has been noted to be suboptimal in some validation data sets [24]. As a result, the Seattle Heart Failure Model (SHFM) was developed and validated as a multivariate risk model to predict 1-, 2-, and 3-year survival in heart failure patients with the use of easily obtainable characteristics relating to clinical status, therapy (pharmacological as well as devices), and laboratory parameters. The SHFM was derived from a cohort of 1,125 heart failure patients in the Prospective Randomized Amlodipine Survival Evaluation (PRAISE1) with the use of a multivariate Cox model. For medications and devices not available in the derivation database, hazard ratios were estimated from published literature. The model was prospectively validated in five additional cohorts totalling 9,942 heart failure patients. The accuracy of the model was excellent, with predicted versus actual 1-year survival rates of 73.4 % versus 74.3 % in the derivation cohort and 90.5 % versus 88.5 %, 86.5 % versus 86.5 %, 83.8 % versus 83.3 %, 90.9 % versus 91.0 %, and 89.6 % versus 86.7 % in the five validation cohorts. Overall receiver operating characteristic area under the curve was 0.729 (95 % CI, 0.714–0.744). The model also allowed estimation of the benefit of adding medications or devices to an individual patient’s therapeutic regimen. The authors concluded that the SHFM provides an accurate estimate of 1-, 2-, and 3-year survival with the use of easily obtained clinical, pharmacological, device, and laboratory characteristics [24].
In a study by Kalogeropoulos and colleagues [25], the SHFM was utilized to predict a composite end point of death, left ventricular assist device (LVAD), and urgent transplantation. However, 98 % of the events in the original SHFM study were death. This fact raises important issues. A higher rate of LVAD implantation and/or urgent transplantation might lead to higher overall event rate. Considering that this patient population was sicker as compared with the original SHFM cohort, it is not surprising that a larger proportion of patients underwent these procedures in their study (16 % vs. 2 %). Thus, miscalibration might not be due to SHFM performance but rather to the SHFM being more accurate for mortality prediction alone rather than a combined outcome. Indeed, when assessing the model performance restricting the outcome to death alone, the model performance improved significantly. Unlike mortality, the timing for urgent transplantation or LVAD implantation can vary between institutions and physicians. With regard to race-based differences, the SHFM needed to be recalibrated by using race-specific coefficients (0.77 for whites and 1.15 for blacks, as estimated in this cohort).
Nonsurgical Management of Heart Failure
Recompensation
The evolution of treatment options for advanced HF patients over the last several decades has been impressive. It includes medical therapies (positive inotropes, vasodilators, angiotensin-converting-enzyme inhibitors and angiotensin-receptor blockers, β-blockade, aldosterone antagonists), defibrillator implantation, resynchronization therapy, heart transplantation, and most recently MCSDs. The comparison of outcomes between different therapies for advanced HF has been challenging. For example, heart transplantation has never been tested in a randomized clinical trial because of the obvious survival advantage in the 1970s in comparison to medical therapy. It is unclear whether this remains true with the recent improvement in HF therapies. Moreover, MCSD is rapidly evolving with advances in technology leading to smaller devices with decreased morbidity. Therefore, the clinical decision-making algorithm is subject to continuing debate and consensus processes, as exemplified by the guideline development initiative of the International Society for Heart and Lung Transplantation [26].
Neurohormonal Blockade
In increasing stages of HF, the adrenergic system, renin-angiotensin-aldosterone system (RAAS), antidiuretic hormone system, and the atrial natriuretic peptide system are chronically activated. These chronic neurohormonal changes lead to compensatory elevation of preload, heart rate, contractility, and cardiac hypertrophy. NYHA class IV is characterized by a flattening and rightward shift of the cardiac function curve to a point where reduced cardiac output does not fulfill the metabolic requirements of the body and capillary wedge pressure reaches a level at which pulmonary edema ensues or both happen [27].
Positive Inotropes/Vasodilators
In the context of refractory acute HF, characterized by peripheral hypoperfusion, renal dysfunction, and marked hypotension present in less than 10 % of acute decompensated HF patients, inotropic agents (classically β-adrenergic agonists and phosphodiesterase inhibitors) have been used as a short-term bridge to cardiac surgery, transplantation, or prolonged infusions via improvement of central hemodynamics. The goals outlined for the utilization of inotropes are as follows: (1) provide rapid relief of congestive symptoms and (2) restoration of end-organ perfusion. If the myocardial insult is deemed reversible, inotropic therapy can be transitioned to organ-saving options. However, if end-organ perfusion cannot be achieved, then mechanical circulatory support (e.g., intra-aortic balloon pump) may be required to transition to possible urgent ventricular assist device or heart transplant [28]. A stepwise approach to the use of inotropic therapy is outlined in Fig. 2.3.
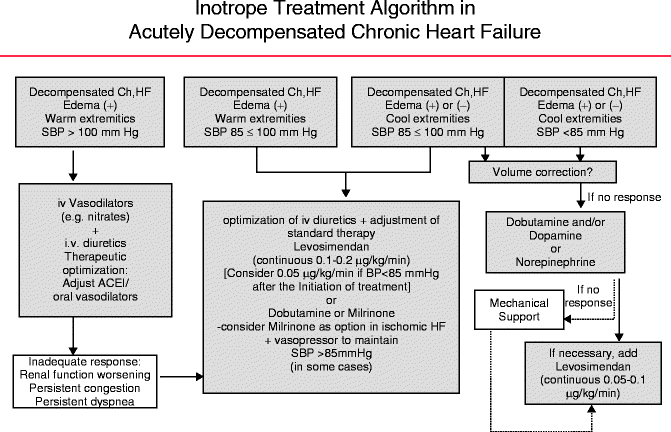

Fig. 2.3
Stepwise approach to use of inotrope therapy
Inotropic agents increase myocardial contractility via increase in intracellular cyclic adenylate monophosphate levels (cAMP). This results in an increase in calcium release from the sarcoplasmic reticulum, thereby increasing the contractile force generation. The phosphodiesterase inhibitors such as milrinone and enoximone inhibit phosphodiesterase III, the enzyme that catalyzes the breakdown of cAMP, whereas the β-adrenergic agonists such as dobutamine and dopamine stimulate adenylate cyclase which increases cAMP production. Dopamine has a dose-dependent mechanism of action: ≤2 mcg/kg/min (dopaminergic receptor activity), 2–5 mcg/kg/min (β-adrenergic receptor activity), and ≥5 mcg/kg/min (alpha adrenergic agonist activity). Both milrinone and dobutamine have similar overall hemodynamic effects with key potential distinctions. Milrinone appears to lower filling pressures to a greater extent than dobutamine. It also has a more profound effect of lowering systemic vascular resistance and blood pressure. On the other hand, dobutamine may result in tachycardia with higher heart rates than milrinone [29]. Therefore, the individual clinical setting should dictate which type of inotrope is used (Table 2.1).
Table 2.1
Inotrope selection in various clinical settings
Clinical scenarios | Inotrope |
|---|---|
Hypotension | Dobutamine or dopamine |
Increased mean pulmonary artery pressure | Milrinone |
Tachycardia | Milrinone |
Renal hypoperfusion | Dopamine, dobutamine, or milrinone |
Despite short-term hemodynamic and symptomatic improvement, long-term mortality appears to be increased with the use of intravenous and oral inotropes for the treatment of chronic heart failure. Positive inotropes such as vesnarinone [30–34] and vasodilators such as epoprostenol did not demonstrate a survival benefit and, in fact, showed an adverse mortality effect [35]. Over the past years, a large clinical development program with the phosphodiesterase III inhibitor, enoximone, yielded promising preliminary results in the phase II results of Oral Enoximone in Intravenous Inotrope-Dependent Subjects (EMOTE) [36]. However, the phase III studies of Oral Enoximone Therapy in Advanced Heart Failure (ESSENTIAL) trial demonstrated a lack of statistically significant differences in time to all-cause mortality or cardiovascular hospitalization [37].
A novel class of inotropic drugs known as calcium-sensitizing agents, such as levosimendan, had generated excitement due to their ability to induce contractility via enhanced troponin C affinity for calcium and stabilization of the calcium-induced conformation of troponin C. The two phase III trials on levosimendan, “Survival in Patients with Acute Heart Failure in Need of Intravenous Inotropic Support” (SURVIVE) [38] and “Second Randomized Multicenter Evaluation of Intravenous Levosimendan Efficacy Versus Survival in the Short-Term Treatment of Decompensated Heart Failure” (REVIVE-II) [39], demonstrated that levosimendan was superior to dobutamine or placebo, respectively, regarding clinical improvement and neurohormonal modulation but failed to demonstrate superiority with regard to 6-month mortality.
Another potential intravenous therapy which promotes vasodilation, salt and water excretion, and improved diastolic filling properties in order to relieve congestion and reduce cardiac filling pressures is nesiritide, a recombinantly produced intravenous formulation of human B-type natriuretic peptide. Rapid and sustained beneficial hemodynamic effects of nesiritide were demonstrated by Mills et al. [40] in NYHA class II–IV patients over a 24-h infusion period and 4 h post-infusion. Effects on clinical outcomes beyond improvement in symptoms and hemodynamics are not clear. In a meta-analysis [41], Sackner-Bernstein and coworkers expressed the opinion that the use of nesiritide could increase the risk of short-term (30-day) mortality. The three trials included in their analysis were the Nesiritide Study Group Efficacy Trial (NSGET) [42], Vasodilation in the Management of Acute Congestive Heart Failure (VMAC) [43], and the Prospective Randomized Outcomes Study of Acutely Decompensated Congestive Heart Failure Treated Initially as Outpatients with Nesiritide (PROACTION) [44]. Another meta-analysis showed that the cumulative short-term (30 days) and long-term (180 days) mortality in patients who received nesiritide combined with or without the use of inotropes [45] was not statistically increased [46].
Based on this conflicting meta-analysis data, an international, multicenter, randomized, double-blind, placebo-controlled study, the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF), has assessed the safety and efficacy of nesiritide. ASCEND-HF randomized 7,141 patients hospitalized with acute HF within 24 h of hospitalization to receive IV nesiritide or placebo in addition to standard therapy. Although there was a trend toward improvement in dyspnea (measured on the 7-point Likert scale) at 6 and 24 h with nesiritide, the prespecified level for significance was not met. Further, there was no difference between the composite end point of 30-day death and HF hospitalization. It was also shown that nesiritide had no impact on worsening of renal function, which had been a prior concern. The authors concluded that nesiritide cannot be recommended for routine use in patients with acute decompensated HF [47].
Adjunctive intravenous therapy which targets the elevated vasopressin (AVP) levels that activate vasoconstriction and left ventricular hypertrophy/remodeling via V1A/V1B receptors and water retention via V2 receptors have also been studied. Both these mechanisms contribute toward acute decompensation of HF. The utilization of intravenous conivaptan, an AVP-receptor antagonist which binds to both V1A and V2 receptors, has demonstrated favorable changes in hemodynamics, with statistically significant reduction in pulmonary capillary pressure and right atrial pressure, and urine output without affecting blood pressure or heart rate [48]. The Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial randomized 4,133 patients hospitalized with HF to oral tolvaptan (selective for V2) or placebo, in addition to standard therapy. Although tolvaptan improved dyspnea, body weight, and edema, there was no significant difference in all-cause mortality or the composite end point of cardiovascular death or HF hospitalization [49]. Thus, vasopressin receptor antagonists may be considered in the management of refractory hyponatremia in HF patients but has no impact on mortality.
RAAS Blockade
Multiple studies have demonstrated the benefit derived from renin-angiotensin system blockade via angiotensin-converting-enzyme inhibitors (ACE-I), including improvements in symptoms, survival, rate of hospitalization, and reverse remodeling. ACE-I decrease the conversion of angiotensin I to angiotensin II, thereby reducing the maladaptive effects of angiotensin II. Furthermore, there is a decrease in the breakdown of bradykinin which promotes vasodilation in the vascular endothelium and promotes natriuresis [7]. At this time, it is unclear if all the different ACE-I demonstrate a similar extent of survival benefit. There is conflicting results from meta-analysis [50], observational studies [51], and comparative trials [52–54]. Moreover, low- versus high-dose enalapril has been studied with no significant differences in survival or clinical and hemodynamic variables [55].
With regard to trial data, the first randomized prospective medical trial demonstrating a survival benefit with ACE-I from a medical treatment in advanced heart failure was the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS-I) trial [56]. Two hundred fifty-six patients in NYHA class IV heart failure were randomized to enalapril or placebo. This trial demonstrated improved survival in the enalapril cohort. This study is unique in being the first heart failure trial in unselected NYHA class IV patients but also in examining extended survival, with sustained benefit for at least 4 years [57]. The subsequent Studies of Left Ventricular Dysfunction (SOLVD) study in 1991 randomly assigned 2,569 patients with symptomatic NYHA class II to III HF and ejection fraction ≤ 35 % to either placebo or enalapril, with reduction in all-cause mortality in the enalapril cohort [58].
Despite the inhibition of the angiotensin-converting enzyme with ACE-I, there is evidence of increased plasma levels of aldosterone. Aldosterone has pleiotropic effects, resulting in increased sodium retention, constriction of systemic arterioles, stimulation of cytokine production, inflammatory-cell adhesion, activation of macrophages as well as stimulation of growth of fibroblasts, and the synthesis of type I and III fibrillar collagens involved in scar formation [59]. Mortality reduction was noted with the addition of aldosterone inhibitors, as evidenced by the Randomized Aldactone Evaluation Study (RALES) trial, in which 1,663 NYHA class III–IV HF patients who had severe heart failure and a left ventricular ejection fraction (LVEF) of ≤ 35 % and who were being treated with an ACE-I, a loop diuretic, and in most cases digoxin were randomly assigned to receive 25 mg of spironolactone daily or placebo. After a mean follow-up period of 24 months, there was a 46 % mortality rate in the placebo group and a 35 % mortality rate in the spironolactone group [60]. The Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study (EPHESUS) trial demonstrated that eplerenone also significantly reduces mortality in post-myocardial infarction (MI) patients with HF or diabetes mellitus with LVEF ≤40 % [61]. More recently, the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS-HF) trial studied eplerenone in HF patients with LVEF ≤30 % (or 30–35 % if QRS duration ≥130 ms) with milder NYHA class II symptoms. In this population, aldosterone antagonism was also associated with improved survival [62].
Another class of medication utilized in RAAS blockade is angiotensin II type 1 receptor blockers (ARB). In the Valsartan Heart Failure Trial (Val-HeFT) study, valsartan significantly reduced the combined end point of mortality and morbidity and improved clinical signs and symptoms in patients with heart failure compared to placebo. This difference was predominantly driven by a 24 % reduction in the rate of HF hospitalizations, without a clear benefit for survival alone. However, the post hoc observation of an adverse effect on mortality and morbidity in the subgroup receiving combined valsartan, an angiotensin-converting-enzyme (ACE) inhibitor, and a β-blocker raised concern about the potential safety of this specific combination [63].
Candesartan in Heart Failure Assessment of Reduction in Mortality and Morbidity (CHARM) addressed whether the angiotensin-receptor blocker (ARB) candesartan improved outcomes in HF patients in two complementary parallel trials (CHARM-Alternative, for patients who could not tolerate ACE-I, and CHARM-Added, for patients who were receiving ACE-I). NYHA II–IV HF patients with LVEF of ≤ V40 % were randomized to candesartan or placebo. The study drug was discontinued in CHARM-Alternative because of adverse effects in 23.1 % of patients in the candesartan group and 18.8 % in the placebo group; the reasons included increased creatinine, hypotension, and hyperkalemia. The authors concluded that candesartan significantly reduces all-cause mortality, cardiovascular death, and heart failure hospitalizations in patients with HF and LVEF ≤ F40 % when added to standard therapies including ACE-I, β-blockers, and an aldosterone antagonist. However, routine monitoring of blood pressure, serum creatinine, and serum potassium is warranted [64]. Thus, ARB are a reasonable alternative to ACE inhibitors as first-line agents for HF. ARB or ACE-I are useful to prevent HF in selected stage A and B patients, and candesartan can improve outcomes in patients with impaired cardiac function who are intolerant of ACE-I [64].
Other landmark trials including Evaluation of Losartan in the Elderly (ELITE II) [65], Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL) [66], and Valsartan in Acute Myocardial Infarction (VALIANT) [67] that have assessed ARB in comparison to ACE-I for treatment of HF have shown no clear benefit of one pharmacologic agent over the other for mortality in HF-REF patients. Studies that have looked at the addition of ARB to background therapy, including the aforementioned Val-HeFT [63], CHARM-Added [68], and VALIANT [67], that already includes ACE-I have also not shown any clear benefit of ARB in addition to ACE-I in reducing mortality in HF-REF.
β-Adrenergic Blockade
The cornerstone of heart failure treatment is neurohormonal blockade of the RAAS and adrenergic systems. According to the European guidelines, ACE inhibition is the first line of therapy, with the initiation of β-blockers (BB) once the patient is clinically stable and ACE inhibitors have been optimized. This paradigm of treatment has resulted in some degree of controversy, pertaining to whether adrenergic blockade should be the front-runner in medical therapy as opposed to ACE inhibition due to its greater impact on sudden death and its initial presence in the sequence of maladaptive neurohormonal activation [69].
The Carvedilol and ACE-Inhibitor Remodeling Mild Heart Failure Evaluation (CARMEN) and the Cardiac Insufficiency Bisoprolol Study (CIBIS) III studies challenged the concept of ACE inhibitors as first-line treatment in CHF. CARMEN explored the need for combined treatment of ACE-I and β-blocker, as well as the order of introduction of these therapies in HF patients with mild, chronic symptoms. They found that combination therapy is superior to ACE-I alone for left ventricular (LV) remodeling as assessed by LV end-systolic volume index on transthoracic echocardiography. When assessing whether introduction of enalapril or carvedilol first had an impact on outcomes, they found that introduction of carvedilol first had a nonsignificant trend toward benefit. The authors concluded that introduction of beta-blockade should not be delayed [70]. CIBIS III was designed to assess the effectiveness of bisoprolol for 6 months followed by combination therapy with enalapril compared to enalapril for 6 months followed by combination therapy with bisoprolol. HF patients with stable mild to moderate symptoms demonstrated non-inferiority of initial initiation of bisoprolol or enalapril in only the intention-to-treat sample for a combined end point of all-cause mortality or hospitalization. However, there was notably more frequent HF events (defined as requiring hospitalization or occurring in the hospital) observed in the bisoprolol group [71]. As a result, first-line treatment with either ACE inhibitors or BB should be based on personalized medicine.
The Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) study group investigated whether metoprolol succinate controlled release/extended release (CR/XL) once daily, in addition to standard therapy, would lower mortality in patients with decreased ejection fraction (EF) and HF symptoms. The study randomized approximately 2,000 NYHA class II–VI patients with chronic HF and with LVEF ≤40 % to either metoprolol succinate or placebo. All-cause mortality, sudden death, and death from worsening HF were lower in the metoprolol group [72].
The CIBIS study group investigated the efficacy of bisoprolol, a β1-selective adrenoceptor blocker, in decreasing all-cause mortality in chronic HF. In a multicenter trial in Europe, they randomized 2,647 NYHA III–IV patients with LVEF ≤35 % receiving standard therapy with diuretics and ACE-I to bisoprolol or placebo. CIBIS-II was stopped early because bisoprolol showed a significant mortality benefit. Treatment effects were independent of the severity or cause of heart failure. The authors concluded that β-blocker therapy had benefits for survival in stable heart failure patients [73].
The Carvedilol Prospective Randomized Cumulative Survival (COPERNICUS) trial demonstrated the beneficial effects of carvedilol, a mixed β1-, β2-, and α1-blocker, on mortality in NYHA class IV patients with chronic HF, with reduction in 1-year mortality from 19.6 % to 11 %, when compared to placebo. All subgroups including those with the most advanced HF showed the same beneficial direction of effect [74]. The Carvedilol or Metoprolol European Trial (COMET) reported a significant survival benefit for carvedilol when compared to metoprolol tartrate in patients with mild-to-severe chronic heart failure [75]. However, the implications of COMET are not fully clear, as critics have argued that the target dosing of metoprolol tartrate (50 mg twice daily) and carvedilol (25 mg twice daily) was not equivalent, with the carvedilol dose being substantially higher [76]. Further, others have argued that long-acting metoprolol succinate should have been directly compared to carvedilol rather than the shorter-acting metoprolol tartrate to achieve more steady-state β-blockade over each 24-h period.
Oral Vasodilators
Hydralazine increases intracellular cyclic guanosine monophosphate (cGMP) to promote smooth muscle relaxation, primarily in the arterioles with reduction in afterload. Nitrates act on the nitric oxide pathway to activate guanylate cyclase and increase cGMP, with predominant venodilation at low doses and vasodilation at higher doses. The original Vasodilator-Heart Failure Trial (V-HeFT) study randomized HF-REF patients who were on background digoxin and diuretic to receive additional therapy with placebo, prazosin (α1-blocker), or combination of isosorbide dinitrate-hydralazine (ISDN-HYD). They found that mortality was lower in the ISDN-HYD cohort compared to placebo at 2 years. Prazosin demonstrated no benefit compared to placebo. Thus, it appeared that ISDN-HYD has potential benefit in chronic HF [77]. However, it should be kept in mind that these patients were not on a background therapy of ACE-I and β-blockade. Subsequently, V-HeFT II randomized 804 patients to either ISDN-HYD or enalapril on background therapy of digoxin and diuretics. The study showed that enalapril resulted in significantly improved survival compared to ISDN-HYD in HF patients [78].
However, there appeared to less benefit of ACE-I compared to ISDN-HYD in African American patients in V-HeFT II. This led to the African American Heart Failure Trial (A-HeFT), which randomized 1,050 NYHA class III–VI HF patients self-described as African American to fixed-dose ISDN-HYD or placebo in addition to standard background therapy that included neurohormonal blockade (including ACE-I, ARB, β-blockers, aldosterone antagonists on the discretion of their regular physicians). The study was terminated early due to significantly improved survival in the ISDN-HYD arm. ISDN-HYD was also associated with improved quality of life. This suggests that there are additional mechanisms of heart failure progression, perhaps decreased NO bioavailability not treated by standard neurohormonal blockade, which are favorably impacted by combined ISDN-HYD [79].
Pulmonary hypertension (PH) is present in 68–78 % of patients with chronic severe LV systolic dysfunction (LVSD) and is commonly associated with right ventricular (RV) dysfunction. Pulmonary vascular resistance (PVR) and RV performance are important determinants of exercise capacity and prognosis in patients with LVSD. The hypothesis that sildenafil, an effective therapy for pulmonary arterial hypertension, would lower PVR and improve exercise capacity in patients with HF complicated by PH was tested in a group of 34 symptomatic HF patients with PH. The patients were randomized to 12 weeks of treatment with sildenafil (25–75 mg orally three times daily) or placebo. Patients underwent cardiopulmonary exercise testing before and after treatment, with greater improvement in pVO2 for the sildenafil group. Sildenafil reduced PVR and increased cardiac output with exercise without altering pulmonary capillary wedge or mean arterial pressure, heart rate, or systemic vascular resistance. The ability of sildenafil to augment pVO2 correlated directly with baseline resting PVR and indirectly with baseline resting right ventricular ejection fraction (RVEF). Sildenafil also improved 6-min walk distance and Minnesota Living with Heart Failure score. The sildenafil cohort experienced fewer HF hospitalizations but had a higher incidence of headache without incurring other serious adverse events. Thus, phosphodiesterase 5 inhibition with sildenafil may improve exercise capacity and quality of life in patients with systolic HF with secondary PH [80].
Antiarrhythmic Therapy
Despite a steady decline in the risk of death from pump failure, many patients remain at high risk for sudden cardiac death (SCD). It accounts for one third to one half of the deaths in patients with HF [81]. Severity of HF is associated with higher overall mortality and higher rate of SCD [72]. Patients with HF are at risk of ventricular arrhythmias, ranging from asymptomatic ventricular premature beats to sustained ventricular tachycardia (VT) or ventricular fibrillation (VF), which can develop into malignant form and can lead to SCD. Some studies have shown arrhythmias not to be the only cause of SCD [82]. Regardless, prevention of arrhythmias remains the key strategy for reducing the risk of SCD.
Most clinical trials of implantable cardioverter/defibrillator (ICD) therapy have demonstrated the superiority of ICD to conventional medical therapy in reducing overall mortality. Most of the antiarrhythmic medications, along with their antiarrhythmic effect, are associated with pro-arrhythmic effects limiting their use as an adjunct to the ICD therapy. Currently, the only antiarrhythmics considered safe for use in HF patients with ventricular arrhythmias are amiodarone and dofetilide. Early trials with amiodarone including the Grupo de Estudio de la Sobrevida en la Insuficiencia Cardiaca en Argentina (GESICA) trial [83] found a significant benefit to mortality and SCD, while the Veterans Affairs Congestive HF Survival Trial of Antiarrhythmic Therapy (CHF-STAT) trial [84] found no benefit in terms of mortality or SCD. Thus, amiodarone is not routinely used in the absence of significant arrhythmias. Other studies have demonstrated increased mortality with sotalol [85] and dronedarone [86] in HF-REF. Radiofrequency ablation and surgical options can also be considered in selected patient populations. In patients with prior MI, the border zone of the infarct is frequently the site of the reentrant circuit, and these sites are often amenable to ablation.
Since most HF patients receive β-blocker therapy, some studies have shown that using β-adrenergic blockers in patients with reduced systolic function and HF symptoms leads to significant reductions in overall mortality rates, which is in part related to reduced SCD. The reduced rate of SCD was 3.9 % versus 6.6 % in MERIT-HF [72] and 3.6 % versus 6.3 % in CIBIS-II [73].
Implantable Cardioverter/Defibrillator
Because of the survival benefit of ICDs as compared with medical therapy, ICDs are the treatment of choice for the primary and secondary prevention of malignant arrhythmias which lead to SCD.
Secondary Prevention
Based on the results of three major clinical trials: Cardiac Arrest Study Hamburg (CASH) [87], Canadian Implantable Defibrillator Study (CIDS) [88], and The Antiarrhythmics Versus Implantable Defibrillators (AVID) [89], which compared ICD to pharmacologic therapy in SCD survivors and other high-risk patients with sustained VT, patients who have survived SCD or had sustained VT are recommended to get an ICD because of their high risk for the development of malignant arrhythmia and SCD. Similarly, all patients who have syncope with either spontaneous or induced sustained VT also should get an ICD. It is unclear whether all patients with unexplained syncope should undergo ICD placement. According to the Heart Rhythm Society guidelines, ICD implantation is recommended if there is significant LV dysfunction due to non-ischemic cardiomyopathy in patients with unexplained syncope [90]. On the other hand, patients with ischemic cardiomyopathy and LV dysfunction (LVEF ≤30 %) qualify for an ICD even in the absence of syncope [91].
Primary Prevention
In asymptomatic patients, there is a mortality benefit with prophylactic use of ICD therapy. Multicenter Automatic Defibrillator Implantation Trial (MADIT) I was the first trial to show that an ICD has a role in primary prevention of SCD. However, the trial enrolled a subselective cohort of patients with prior MI, nonsustained VT, LVEF ≤35 %, and inducible sustained monomorphic VT [91]. The Multicenter Unsustained Tachycardia Trial (MUSTT) trial showed that patients with prior MI, asymptomatic nonsustained VT, LVEF ≤40 %, and inducible sustained VT had reduced sudden cardiac death with ICD implantation for primary prevention [92]. MADIT II was subsequently carried out to expand the population compared to earlier studies, enrolling patients with LVEF ≤30 % more than 30 days post-MI. Unlike the earlier studies, electrophysiologic testing and presence of nonsustained VT were not required for enrollment. Patients were randomized to ICD or medical therapy, with the trial terminated early due to significant reduction in all-cause mortality for the ICD cohort, due to reduction in sudden cardiac death [93].
The Sudden Cardiac Death in Heart Failure (SCD-HeFT) trial included all HF patients with LVEF ≤35 % and NYHA class II–III, regardless of ischemic or non-ischemic etiology. Patients were randomized to either ICD implantation, amiodarone, or placebo. At 5 years, mortality was significantly improved with ICD therapy in both ischemic and non-ischemic cardiomyopathy. Amiodarone had no impact on survival [94]. The decision to use ICD therapy in asymptomatic patients with non-ischemic cardiomyopathy can be challenging. Different risk prediction methods (e.g., microvolt T-wave alternans) [95] have been used to predict the risk of arrhythmia, without the identification of any clear risk stratifiers. Some patients might die because of arrhythmia despite ICD therapy, which may be related to heart failure severity or frequency of appropriate and inappropriate shocks received from ICD versus no shocks, as was demonstrated from the SCD-HeFT trial results [96].
The most recent AHA/ACC guidelines [97] for primary prevention with ICD recommend implantation for (1) LVEF ≤35 % due to prior MI, who are at least 40 days post-MI and NYHA class II–III; (2) LVEF ≤35 % in non-ischemic dilated cardiomyopathy who are NYHA class II–III; (3) LVEF ≤30 % due to prior MI, who are at least 40 days post-MI and NYHA class I; and (4) LVEF ≤40 % due to prior MI, with nonsustained VT and inducible VF or VT at electrophysiological study.
Cardiac Resynchronization Therapy (CRT)
A growing body of evidence suggests that the use of implantable devices to resynchronize ventricular contraction may be a beneficial adjunct in the treatment of chronic heart failure. One third of patients with chronic heart failure have electrocardiographic evidence of a major intraventricular conduction delay, which may worsen left ventricular systolic dysfunction through asynchronous ventricular contraction. Uncontrolled studies suggest that multisite biventricular pacing improves hemodynamics and well-being by reducing ventricular asynchrony.
The Multisite Stimulation in Cardiomyopathies (MUSTIC) trial showed that CRT in NYHA class III HF-REF patients with QRS ≥150 ms resulted in improvement in 6-min walk distance, quality of life, and pVO2, with reduced hospitalizations [98]. In the Multicenter InSync Randomized Clinical Evaluation (MIRACLE) trial, patients with NYHA class III-IV HF from either ischemic or non-ischemic cardiomyopathy, LVEF ≤35 %, LVEDD ≥55 mm, and QRS duration of ≥130 ms were randomized to CRT or conventional therapy. Patients randomized to CRT had an improvement in 6-min walk distance, quality of life, functional class, time on treadmill during exercise testing, and ejection fraction. Further, CRT reduced hospitalization compared to control [99]. The Comparison of Medical Therapy, Pacing, and Defibrillation in Chronic Heart Failure (COMPANION) trial randomized NYHA class III–IV patients with LVEF ≤35 % and QRS ≥120 ms to receive optimal pharmacologic therapy (diuretics, ACE-I, β-blockers, and spironolactone) alone or in combination with CRT with either a pacemaker or a pacemaker-defibrillator. CRT with either pacemaker or pacemaker-defibrillator resulted in reduction of the primary end point of time to all-cause mortality or hospitalization by 34 % and 40 %, respectively, when compared to pharmacologic-only therapy. The authors concluded that CRT decreases the combined risk of death from any cause or first hospitalization and, when combined with an ICD, significantly reduces mortality [100]. The Cardiac Resynchronization Heart Failure (CARE-HF) study randomized patients with NYHA class III–IV HF, LVEF ≤35 %, and cardiac dyssynchrony to CRT or standard pharmacologic therapy. CRT reduced time to all-cause mortality or cardiovascular hospitalization [37], with reduction in mortality that persisted to an extended follow-up of 38 months [101]. Further, CRT reduced the interventricular mechanical delay, the end-systolic volume index, and the area of the mitral regurgitant jet; increased the LVEF; and improved symptoms and the quality of life. The authors concluded that in patients with heart failure and cardiac dyssynchrony, cardiac resynchronization improves symptoms and the quality of life as well as reducing complications and the risk of death. The beneficial effects of CRT in this group of patients were impressive, considering that these patients were receiving optimal medical therapy with diuretics, β-blockers, spironolactone, ACE-I, or ARB at the time of enrollment. The results showed that for every nine devices implanted, one death and three hospital stays were prevented [37].
Other studies have explored the use of CRT in patients with milder HF symptoms. Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) demonstrated that in NYHA class I–II symptoms with LVEF ≤40 % and QRS ≥120 ms, CRT resulted in a reduction in HF hospitalization, with improvement of ventricular structure and function. However, the REVERSE study did not examine the impact of CRT on mortality in these patients with milder HF symptoms [102]. The Resynchronization-Defibrillation for Ambulatory Heart Failure Trial (RAFT) randomized patients with NYHA class II–III HF, with LVEF ≤30 %, intrinsic QRS ≥120 ms, or paced QRS ≥200 ms to ICD alone compared to ICD plus CRT. With CRT, there was a reduction in a combined end point all-cause mortality or HF hospitalization. Independently, there was a reduction in mortality alone. However, there was increased rate of device-related complications in the CRT cohort [103]. The recent MADIT-CRT trial explored the use of CRT in patients with NYHA class I–II HF, LVEF ≤30 %, and QRS ≥130 ms, showing a reduction in a composite of all-cause mortality and nonfatal HF event, but was predominantly driven by a 41 % reduction in risk of HF events. There was no difference in risk of death alone [104]. The 2012 AHA/ACC class I recommendation for CRT includes patients with LVEF ≤35 %, sinus rhythm, left bundle branch block (LBBB) morphology with QRS ≥150 ms, and NYHA class II–IV symptoms. Class IIa indications include expanded criteria including LBBB with QRS duration of 120–149 ms, non-LBBB with QRS ≥150 ms, and in patients with atrial fibrillation if they require ventricular pacing [105].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


