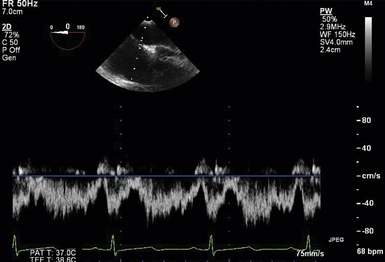
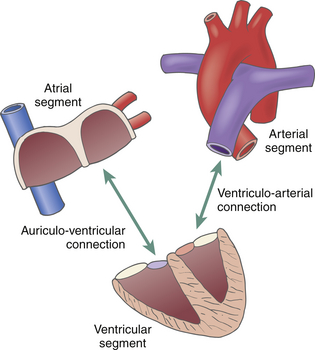
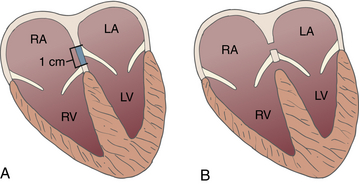
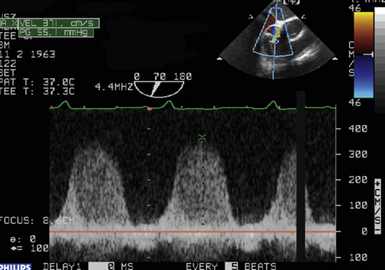
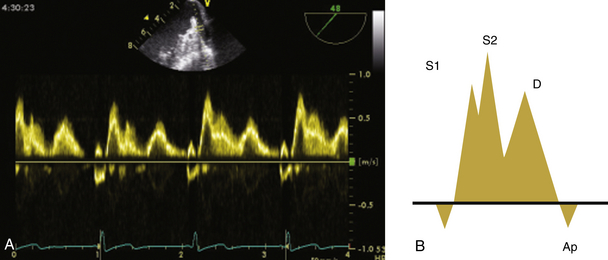
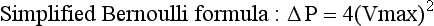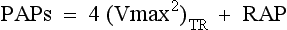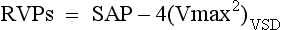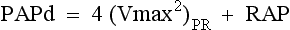24 Congenital heart disease (CHD) affects an estimated 0.5% to 1% of all live births.1–3 Thanks to major advances in congenital cardiac surgery made over the past few decades, the majority of these patients now survive to adulthood, even those with complex CHD lesions. The number of adult survivors with CHD has thus constantly increased over the past decades, and their number now exceeds the number of affected children. 4 It is, however, important to realize that after childhood repair of CHD, the majority of these adult survivors are not “cured”; many of them remain at risk for complications and the need for reoperations. There are three groups of “grown-up CHD” (GUCH) patients that may be encountered: 1. Patients after intracardiac repair of congenital cardiac defects in childhood. Only a few of these patients (i.e., repair of atrial septal defects [ASDs] or patent ductus arteriosus in early childhood) can be regarded as cured. The majority of these patients, particularly those after repair of moderate or complex forms of CHD, are at risk for late complications due to residual hemodynamic lesions and myocardial scars. 2. Patients with unrepaired CHD amenable to intracardiac repair in adulthood. This group consists mainly of simple lesions such as different forms of ASDs, isolated aortic or pulmonary valve disease, or coarctation of the aorta. This group, however, also includes patients with more complex diseases such as Ebstein anomaly of the tricuspid valve, unrepaired tetralogy of Fallot (TOF), or corrected congenital transposition of the great arteries (TGA). 3. Patients with complex defects without prior surgery or patients after palliative surgery for complex congenital cardiac malformations. This group includes patients after Fontan palliation for single-ventricle physiology and patients after systemic-to-pulmonary shunt operations (i.e., Blalock-Taussig shunts) in the setting of complex CHD. 1. Evaluation of complex CHD, particularly in patients in whom the preoperative evaluation is incomplete by transthoracic echocardiography (TTE) 2. Assessment of anatomic abnormalities and their surgical repair during cardiac interventions 3. Management of hemodynamics during cardiac and non-cardiac surgery, extending into the postoperative intensive care unit (ICU) TEE during cardiac surgery for CHD is considered a category I indication.5,6 The principal uses of TEE in the perioperative or peri-interventional setting may be classified under three headings ( Box 24-1): 1. In the catheterization laboratory, TEE is often used to guide catheter-based intracardiac procedures. It is particularly useful for guidance of device closure in patients with secundum-type ASDs. TEE provides precise information on location, geometry, and number of ASDs, as well as the extent of surrounding tissue and location of adjacent structures. 7 It allows delineation of pulmonary venous return and allows exclusion of interatrial defects not amenable to device closure (i.e., sinus venosus defects). It is important to emphasize that periprocedural TEE should not stand alone as the sole diagnostic study, since there are inherent limitations in imaging certain important structures that are best identified by TTE. 8 In case of discrepant findings from the preinterventional diagnosis, mutual assessment with colleagues from cardiology is advisable. 2. In the operating room, TEE is helpful during all stages of cardiac surgery. 3. In the ICU, it is particularly important in the assessment of hemodynamically unstable patients. The echocardiographer needs to pay attention to the hemodynamic status prior to and during the procedure. For example, mitral valve function is highly dependent on left ventricular afterload; when assessing severity of mitral regurgitation, it is of utmost importance to reestablish normal systemic vascular resistance, if necessary by administration of arterial vasopressors. Assessment of the magnitude of residual intracardiac shunting may be difficult. First, the pulmonary arterial pressure, if elevated preoperatively, may not fall immediately after separation from cardiopulmonary bypass (CPB), resulting in underestimation of the severity of a potential residual left-to-right shunt. Second, many patients require increased inspired oxygen concentration during the evaluation, which may provide a spurious error in shunt calculations. Third, although color Doppler is an excellent tool for localizing residual shunt lesions, it is unreliable for determining absolute shunt size, particularly when it is located in the muscular ventricular septum. 9 The impact of TEE in cardiac surgery for CHD has been mostly studied in the pediatric population. The rate of new findings and/or surgical management alterations based on intraoperative TEE ranges between 3% and 39%.10–15 Sensitivity and specificity of TEE to determine the necessity for reoperation reaches 89% and 100%, respectively.16,17 A major impact of TEE, defined as new information altering the planned procedure or leading to a revision of the initial repair, occurs in 13% to 16% of cases12,13 and is most frequently seen during reoperations, valve repairs, complex AV discordances, and complex outflow tract reconstructions.11,12 Patients who leave the operating room with significant residual defects or decreased ventricular performance tend to have a poorer outcome.13,18,19 It has been recognized that residual anatomic or functional lesions are the main determinants of morbidity and mortality after repair of congenital cardiac defects.19,20 Therefore, intraoperative TEE is used more and more frequently in congenital cardiac surgery to assess operative results. Assessing the immediate result of surgical repair is of great importance. Return to CPB based on findings from intraoperative TEE is reported in 5% to 11% of cases.10,11,14,16,21–24 After weaning from CPB, a return to bypass is always a difficult decision for the surgical team, particularly after a long and complicated procedure. It is thus essential to keep in mind that the goal of surgery is to obtain a good clinical result but not a perfect echocardiographic image. The echocardiographer must understand all the implications of a return to bypass. The decision may be easy in the case of hemodynamic compromise, but it requires careful consideration in the absence of hemodynamic instability. Optimal collaboration and communication between the echocardiographer and the surgical team in making the best decision regarding management of an individual patient is important. 9 The intraoperative surgical revision rate may decrease over time with experience, as has been shown by Ungerleider et al., who reported a decrease in rate from 8.5% to 3% to 4% over a 7-year period with the same surgeon. 18 It supports the notion that institutional surgical skill and echocardiographic experience will directly affect the impact of TEE. On the other hand, the rate of return to bypass for incomplete repair decreases from 9.6% to 0% when an experienced perioperative echocardiographer is replaced by a poorly trained echocardiographer, and the rate of missed residual problems after bypass rises from 21% to 74% 25; these findings reinforce the importance of good collaboration between echocardiographer and surgical team and particularly the need for a well-trained and experienced echocardiographer. The diversity of GUCH requires a structural classification. The most useful concept is the segmental approach26,27: the heart is divided into three segments (atria, ventricles, and arterial trunks) connected via two junctions (atrioventricular and ventriculo-arterial) ( Fig. 24-1). The definition of the segments is based on their intrinsic morphology, because the usual criteria (e.g., size, position of cardiac chambers) may be altered in CHD. Although most patients entering the operating room have been previously assessed and a diagnosis made, it is important to understand the principles of this classification to allow unambiguous communication between the echocardiographer and the surgical team. Sequential analysis of the heart includes five steps to describe cardiac anatomy. 28 Figure 24-1 Schema of segmental approach: heart is divided into three segments (atria, ventricles, and arterial trunks) and two junctions (atrioventricular and ventriculo-arterial junctions). (Modified from Bettex D, Chassot PG. Transesophageal echocardiography in congenital heart disease. In: Bissonnette B, ed. Pediatric Anesthesia: Basic Principles—State of the Art—Future. Shelton, CT: People’s Medical Publishing House-USA; 2011, with permission.) Atrial situs describes the morphology and arrangement of the atria. It can be normal (situs solitus), mirror imaged (situs inversus), or ambiguous in the setting of right or left atrial isomerism. Most often but not universally, abdominal and thoracic situs follows cardiac situs. The most important features in distinguishing the right and left atria are their appendages. Apart from its broad-based appendage, the right atrium (RA) has several morphologic characteristics that allow its differentiation from the left atrium (LA). The inferior vena cava (IVC) is almost uniquely connected to the RA. The eustachian valve boards this connection. Another typical and unique structure of the RA is the crista terminalis, separating the smooth-walled sinus venarum (entry of the IVC, superior vena cava [SVC], and coronary sinus [CS] into the RA) from the trabeculated RA. The LA has very few characteristic structures other than a typically finger- or hook-shaped appendage bordered by pectinate muscles, which should not be confused with a thrombus. The left ventricle (LV) is defined by the presence of one or two papillary muscles, fine apical trabeculations, usually a bicuspid mitral valve, and a partially fibrous outlet. The mitral valve is in fibrous continuity with the outflow tract, and there is no infundibulum. The right ventricle (RV) is defined by three or several papillary muscles (one of which inserts into the interventricular septum), a tricuspid valve with its septal leaflet inserted more apically than the anterior mitral valve leaflet ( Fig. 24-2), coarse apical trabeculations, and a completely muscular outlet or infundibulum. Figure 24-2 Schema of implantation of mitral and tricuspid valves. A, Normal anatomy: tricuspid valve is inserted lower than mitral valve at level of interventricular septum. B, Atrioventricular canal: mitral and tricuspid valves are inserted at same level of interventricular septum. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. (Courtesy PG Chassot.) Concordant atrioventricular (AV) connection means that the RA is connected to the RV and the LA to the LV. In the case of discordant AV connection, the RA is connected to the LV and the LA to the RV. Variants include a common AV junction in the setting of atrioventricular septal defects (AVSDs) (see later), overriding of one of the AV valves (<50% of the valve opens into the contralateral ventricle) ( Fig. 24-3), or a double inlet ventricle (>50% opens into the contralateral ventricle). Straddling of the AV valve is defined by the presence of chordal attachments crossing through an interventricular septal defect and inserting into the contralateral ventricle (see Fig. 24-3). This has important implications for surgical repair because it usually does not allow biventricular repair. Figure 24-3 Schema of an overriding and straddling mitral valve. A, Overriding mitral valve: atrioventricular valve opens less than 50% into contralateral ventricle. B, Straddling mitral valve: a straddling valve has chordal attachments crossing through an interventricular septal defect and inserting into opposite ventricle. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle; VSD, ventricular septal defect. (Courtesy PG Chassot.) The ventriculo-arterial connection describes the relationship between the ventricles and the large arterial trunks. It can also be concordant or discordant (TGA). In the presence of a ventricular septal defect (VSD), there can be overriding of an arterial trunk (<50% connection to the opposite ventricle) ( Video 24-1 In a last step, we need to define all associated abnormalities, such as hypoplastic heart chambers, septal defects, obstructive lesions, and valve abnormalities. Given the principles of sequential anatomy outlined earlier, a comprehensive TEE examination in patients with CHD is recommended in a systematic and logical sequence: 29 • Atrial segment: situs, location, arrangement, identity, venous connections • Four-chamber view: relative size, shape, and position of each cardiac cavity • AV connection: valvular status, univentricular, uniatrial, single-inlet • Ventricular segment: number, size, orientation, identity • Ventriculo-arterial connection: double outlet, single outlet, outflow tract, valvular status • Arterial segment: great vessels orientation, identity • Presence and direction of intracardiac and extracardiac shunts Intracardiac shunts may be located at the level of the interatrial septum (ASDs), interventricular septum (VSDs), or between the aorta and atria (aorto-atrial fistula) (Video 24-2 1. Direction and timing of the flow: left to right (L-R), right to left (R-L), or bidirectional. The shunt flow can be systolic, diastolic, or continuous systolo-diastolic. Both flow direction and timing are determined by the pressure difference between the two affected cardiac chambers and/or vessels, which changes over the cardiac cycle. 2. Dimension of the defect: a shunt of small size generates a high-pressure gradient and turbulent flow; a large shunt does not impede blood flow, resulting in low or no gradient and laminar flow. 3. Enlargement of the receiving chambers: isolated defects situated upstream of the AV valves (ASD, anomalous pulmonary venous return) cause right-sided chamber dilation, whereas lesions located downstream of the AV junctions (VSD, ductus arteriosus) induce left-sided chamber dilation. In both cases, the pulmonary artery is dilated and pulmonary blood flow is increased. Systolic pulmonary artery pressure (PAPs) can be estimated from the tricuspid regurgitation (TR) jet velocity ( Fig. 24-4). In the absence of pathology in the right ventricular outflow tract (RVOT), systolic RV pressure is identical to the PAPs. It equals the pressure gradient between RV and RA, which can be calculated by the simplified Bernoulli equation with the addition of the RA pressure (RAP): Figure 24-4 Continuous wave Doppler spectrum of tricuspid valve regurgitation. Using Bernoulli equation, peak velocity gives us peak gradient between right atrium (RA) and right ventricle (55 mmHg). Assuming RA pressure of 14, pulmonary systolic pressure, in absence of any right ventricular outflow tract pathology, would be: 55 + 14 = 69 mmHg. This formula may be used if there is no obstruction within the left ventricular outflow tract (LVOT); the systolic arterial pressure is then roughly equivalent to the maximal LV systolic pressure. In GUCH, the RV may be the subpulmonic ventricle, supporting pulmonary circulation, but in the setting of transposition complexes, it may be the subaortic ventricle, supporting systemic circulation. In these patients, anatomy and function of the RV has been of interest for quite some time. Before starting to delineate individual congenital cardiac lesions, it may be useful to give a few specific considerations to RV anatomy and physiology. 30 The complex more triangular shape of the RV contrasts with the more conical shape of the LV ( Fig. 24-5). The muscular wall of the normal RV is thin, usually 3 to 5 mm in thickness, but in cases of pressure overload, its thickness may even exceed that of the LV. Contraction of the RV myocardium relies more heavily on longitudinal shortening, and as a subpulmonary ventricle pumping into a low-resistance vascular bed, RV mechanics are quite different from LV mechanics. Although a morphologic RV seems inherently incapable of functioning as a subaortic systemic ventricular pump, it has a remarkable capacity for adaptation and may function at systemic pressures for decades (i.e., congenitally corrected transposition, Senning or Mustard repair for complete TGA). Under these circumstances, RV mechanics resemble those of an LV. Figure 24-5 Anatomy of right ventricle (RV). Endocast of a normal right heart with right heart chambers colored blue and left heart chambers colored red is viewed from different perspectives to display spatial relationships between cardiac chambers. A, Anterior aspect with crossover of left and right ventricular outflow tracts; pulmonary valve (PV) is situated more superiorly. B, From right and anterior: triangular shape. C, From apex: crescentic form, wrapping around left ventricle (LV). Ao, Aorta; Inf, inferior; IVC, inferior vena cava; PT, pulmonary tract; RA, right atrium; Sup, superior. (Modified from Ho SY, Nihoyannopoulos P. Anatomy, echocardiography, and normal right ventricular dimensions. Heart 2006;92:i2-i13, with permission.) Precise and reproducible echocardiographic measurements of the RV are challenging because of its complex shape. 31 It must be imaged in multiple planes, and a qualitative visual assessment is usually applied on TEE. To define the size of the RV, its relative size compared to the LV on four-chamber view is often used as a qualitative measure. Similarly, the systolic RV function is usually characterized as normal, mildly, moderately, or severely abnormal. Rapid advancements in the field of magnetic resonance imaging (MRI) have established this technique as the reference standard for quantitative assessment of RV volumes, mass, and systolic function, regardless of whether the RV is in subpulmonic or subaortic position. The most useful transesophageal views to assess the RV are: 32 • Midesophageal (ME) four-chamber view: visualization of the RV lateral wall and measurement of RV internal dimensions and RV fractional area change • ME RV inflow-outflow view (30-60 degrees): visualization of the coronary sinus, assessment of TR • Upper esophageal (UE) views: visualization of the RVOT, assessment and quantification of pulmonary stenosis or regurgitation • Transgastric (TG) views (0-120 degrees): short-axis views of the RV, anterior and inferior walls of the RV, the septum as well as the RV inflow and outflow tract, the IVC, and the hepatic veins • Deep TG views: visualization of the inflow and outflow tract of the RV, tricuspid annular tissue Doppler signals Volume-based indices of RV function have limitations because of geometrical assumption and load dependency. Other Doppler measurements may add insights into RV function, such as dP/dt of the TR velocity, as well as the index of myocardial performance, or Tei index, of the RV. 33 Other measurements include tissue Doppler imaging (TDI) of the tricuspid annulus and myocardial acceleration during isovolumic contraction, which measure intrinsic contractility but are not used routinely. 30 We differentiate two broad contexts of RV adaptation to CHD: the volume-loaded RV and the pressure-loaded RV. The three most common lesions associated with RV volume overload are the different types of ASDs, significant PR, and TR. The two most common lesions associated with RV pressure overload are different forms of RVOT obstruction and cases in which the RV serves as the subaortic systemic ventricle. Persistence of the left superior vena cava (LSVC) is the most common anomaly of the systemic venous connection, and in the absence of associated defects may be considered a variant of normal. It is found in 0.5% of the general population and up to 10% of GUCH patients. 34 The LSVC usually drains into the coronary sinus (CS) but may enter the LA directly, leading to a “right-to-left” shunt. When the LSVC drains into the CS, its hallmark on echocardiography is enlargement of the CS. ( Fig. 24-6). On the transverse plane, the LSVC lies close to the lateral wall of the LA between the left upper pulmonary vein and the left atrial appendage. A microbubble injection into an upper left-sided vein follows the drainage into the anomalous system. The right SVC and innominate vein may be absent. Other etiologies may also dilate the CS, like an anomalous connection of the left pulmonary veins to the CS, a coronary fistula, or any lesion producing a marked increase in RA pressure, such as pulmonary hypertension or severe TR; they should be excluded before diagnosing a persistent LSVC. While a LSVC usually is an incidental finding without clinical implications, it may be important in the perioperative setting because it may change central venous or pacemaker accesses as well as, during CPB, the technique for venous cannulation or cardioplegia (retrograde cardioplegia is not possible). Figure 24-6 A, Schema of left superior vena cava (LVSC). Coronary sinus is dilated and visualized in long axis at level of eso-gastric junction. B, Schema of LVSC. In midesophageal (ME) view, dilated vena cava may be seen between left atrial appendage (LAA) and superior pulmonary vein. C, Bidimensional imaging of LVSC in ME view. Ao, Aorta; CS, coronary sinus; LA, left atrium; LUPV, left upper pulmonary vein; LV, left ventricle; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract. (A and B courtesy PG Chassot.) In cases of cannulation of the venae cavae, stenosis at the site of cannulation should be excluded after bypass. With the use of color Doppler, acceleration at the level of the stenosis might be visualized ( Fig. 24-7, A); the normal biphasic pulsed wave Doppler flow through the SVC is then replaced by a continuous flow without return to baseline and with a relatively high velocity (>1.5 m/s) ( Fig. 24-7, B). Some or all of the pulmonary veins may be falsely connected to the RA instead of the LA. Without surgery, patients with total anomalous pulmonary venous return do not survive to adulthood, and the unoperated patient is therefore not encountered in adulthood. In contrast, partial anomalous pulmonary venous return (PAPVR) drainage is encountered in adults, either as an isolated lesion or in combination with other defects (i.e., sinus venosus defects). The most common form is anomalous drainage of the right upper and middle pulmonary vein into the RA or into the base of the SVC. Figure 24-8 depicts PAPVR in the setting of a superior sinus venosus defect. The right lower pulmonary vein may anomalously drain into the IVC, as in Scimitar syndrome. In this case, the interatrial septum is usually intact. Isolated left pulmonary veins may connect through a left-sided vertical vein to the innominate vein or directly to the CS. PAPVR causes L-R shunting that leads to volume overload and dilation of the right-sided heart chambers. Figure 24-8 Superior sinus venosus defect. Midesophageal bicaval view: this type of atrial septal defect (ASD) is mostly associated with a partial anomalous pulmonary vein return. In red, inflow of right ventricle (RV) into superior vena cava (SVC). LA, Left atrium; RA, right atrium; RPV, right pulmonary vein. Since the pulmonary veins return posteriorly into the atria, TEE is superior to TTE for evaluating PAPVR. It is possible to see the veins beyond their left atrial site of connection as far as the hilum of each lung. 9 At least four pulmonary veins draining into the LA must be documented to exclude significant PAPVR. In some patients, even more than four veins may be present (five in 10% of cases 35); in others, left-sided veins in particular may drain together into the LA. Identification of pulmonary venous connections is usually obtained from multiple views. The normal entry of pulmonary veins will be found in the UE four-chamber view at 0 and 90 degrees. If the entry of all the pulmonary veins into the LA are not visualized, anomalous connections should be searched for, especially in the case of an otherwise unexplained dilation of the IVC, SVC, RA, or RV. The exact anatomy and site of drainage of anomalous left pulmonary veins may be missed on TEE because of acoustical interference from the left bronchial tree. After surgical repair of anomalous venous connections, venous flow must be present with a biphasic systolo-diastolic pattern on spectral Doppler ( Fig. 24-9), with a low maximal velocity (usually below 1 m/s) and a return to the baseline between the systolic and diastolic peaks. A continuous nonphasic pattern and a peak velocity of 1.5 m/s or more are indicative of significant residual stenosis (see Fig. 24-7, B). 36 Figure 24-9 Pulmonary venous spectrum Doppler. A, Normal pulsed wave Doppler (PWD) of left superior pulmonary vein (PV). B, Schema of a normal PWD of PV. PV flow has three different parts: systolic component (which might be bifidous), with S1 simultaneous to atrial relaxation and S2 to mitral annulus descent; diastolic component (D) simultaneous to passive filling through mitral valve; and reverse A-wave (Ap) simultaneous to contraction of atrium. Maximal velocity does not exceed 1 cm/s, and flow reaches baseline between systolic and diastolic peaks. ASDs represent about 7% of all CHD and 30% of GUCH. Because these defects often cause few symptoms, diagnosis in adulthood is not uncommon. 37 Ostium secundum–type defects located in the fossa ovalis are most common and account for 60% to 75% of all cases. Ostium primum–type defects are part of the spectrum of AVSDs and account for about 15% of cases. Sinus venosus defects are typically associated with partially anomalous right pulmonary venous connections and account for about 10% of cases. Coronary sinus defects (also named unroofed coronary sinus) are rare defects ( Fig. 24-10). The volume overload secondary to the L-R shunt is proportional to the size of the defect and the ratio between left and right atrial pressures. With progressive stiffening of the LV, a physiologic occurrence of aging resulting in increased LA pressure, the shunt flow usually increases. In large defects, volume load of the RA and RV leads to dilation of these chambers and enlargement of the size of the pulmonary artery. Figure 24-10 Schema of different atrial septal defects. Interatrial septum may present four types of defects: ostium secundum, situated at level of fossa ovalis; ostium primum in atrioventricular septum; sinus venosus at entry sites of venae cavae, frequently associated with abnormal pulmonary venous connection; and coronary sinus defect, an unroofing of coronary sinus into left atrium. (Modified from Bettex D, Chassot PG. Transesophageal echocardiography in congenital heart disease. In: Bissonnette B, ed. Pediatric Anesthesia: Basic Principles—State of the Art—Future. Shelton, CT: People’s Medical Publishing House-USA; 2011, with permission.) The diagnosis by echocardiography is made with 2D imaging (echo dropout area in the interatrial septum) ( Fig. 24-11, A; Video 24-3, A Figure 24-11 Ostium secundum defect. A, Bicaval midesophageal view of an atrial septal defect (ASD) secundum: echo-dropout in middle of interatrial septum. B, Same view with color Doppler: laminar color flow Doppler through ASD secundum, typical of a nonrestrictive defect. C, Three-dimensional imaging of ostium secundum ASD. Tricuspid valve is situated on right side of image. Ao, Ascending aorta; IVC, inferior vena cava; LA, left atrium; RA, right atrium; SVC, superior vena cava.) (C courtesy PG Chassot.) Figure 24-12 A, Midesophageal four-chamber view of ostium primum atrial septal defect (ASDI): echo dropout at base of interatrial septum. B, Same image with color Doppler: laminar color flow typical of nonrestrictive shunt. LA, Left atrium; LV, left ventricle; RA, right atrium; RV, right ventricle. Figure 24-13 Upper esophageal basal view of superior sinus venosus defect: color flow Doppler shows communication between both atria at entrance of the superior vena cava (SVC) into right atrium (RA). Flow is laminar, shunt is nonrestrictive. ASD, Atrial septal defect; LA, left atrium. Since the pressure difference between both atria is usually small, the maximal blood flow velocity across the defect usually varies between 0.5 and 1.5 m/s. The L-R flow presents as a typical biphasic cyclic pattern on spectral Doppler ( Fig. 24-14). Variations of the flow are related to the cardiac cycle: one peak of L-R flow occurs during late systole and early diastole (synchronous with “v” wave), and one peak during the atrial contraction (synchronous with “a” wave). A short period of R-L shunting can usually be recorded during early systole and mid-diastole.39,40 This flow pattern is consistent with the instantaneous cyclic pressure differences between the left and right atria ( Fig. 24-15). 41 The most important shunt flow reversal is observed in protosystole when the mitral annulus descent abruptly increases the LA volume and therefore decreases its pressure. 42 Positive-pressure ventilation (PPV) and positive end-expiratory pressure (PEEP) increase the R-L components of the shunt by augmenting RV afterload. Because of the slower frame rates observed with color flow Doppler, the R-L component of the atrial shunt is usually not detectable on color flow, although it is easily identified on pulsed wave Doppler or with use of agitated saline contrast. The inflow of the IVC may sometimes be confused with an interatrial shunt, particularly in the case of a prominent eustachian valve.
Adult Congenital Heart Disease
![]() Introduction
Introduction
![]() Indications for TEE
Indications for TEE
![]() Impact of TEE
Impact of TEE
![]() Anatomic Nomenclature: The Segmental Approach
Anatomic Nomenclature: The Segmental Approach
Define Atrial Situs or Atrial Arrangement
Define Ventricular Chambers
Define Atrioventricular and Ventriculo-arterial Connections
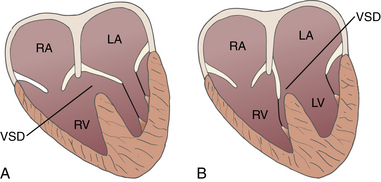
![]() ) or a double outlet ventricle (>50% overriding). In the case of a double outlet right ventricle (DORV), bilateral muscular infundibula are often found, with loss of fibrous continuity between the mitral and the aortic valve.
) or a double outlet ventricle (>50% overriding). In the case of a double outlet right ventricle (DORV), bilateral muscular infundibula are often found, with loss of fibrous continuity between the mitral and the aortic valve.
Associated Anomalies
Shunt and Pressure Gradient
![]() ). Shunts may also be located at the level of the great arteries (aorto-pulmonary window, patent ductus arteriosus) or be caused by partial or complete anomalous pulmonary venous drainage into the RA. A shunt flow can be defined by three characteristics:
). Shunts may also be located at the level of the great arteries (aorto-pulmonary window, patent ductus arteriosus) or be caused by partial or complete anomalous pulmonary venous drainage into the RA. A shunt flow can be defined by three characteristics:
![]() Specific Congenital Cardiac Defects
Specific Congenital Cardiac Defects
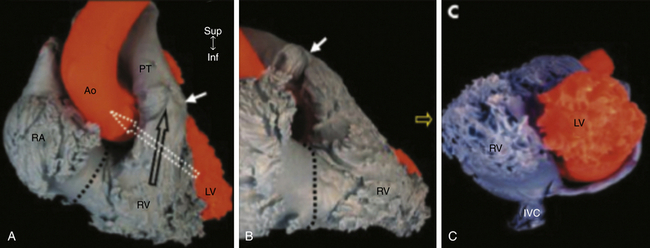
The Right Ventricle in Congenital Heart Disease
Individual Congenital Cardiac Lesions
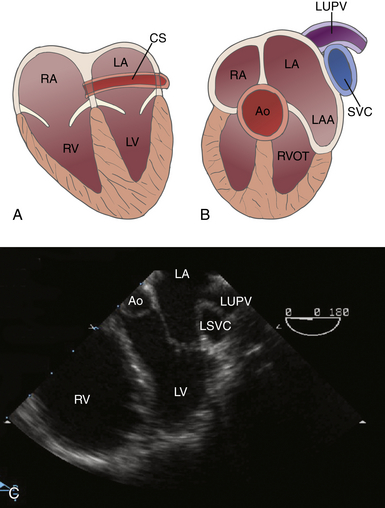
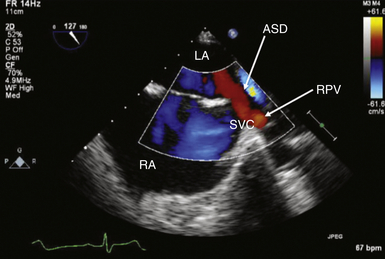
Anomalous Venous Return
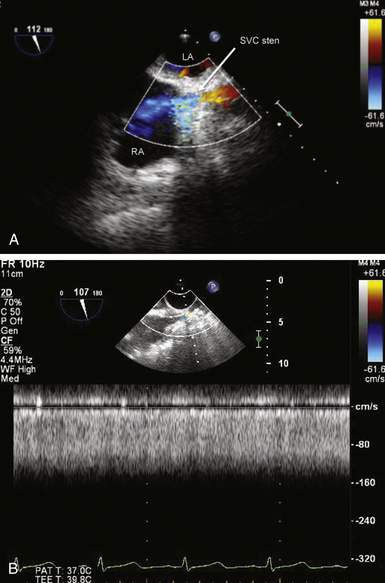
Anomalous Systemic Veins
Anomalous Pulmonary Venous Connections
General Considerations and Preoperative Evaluation
Postoperative Evaluation
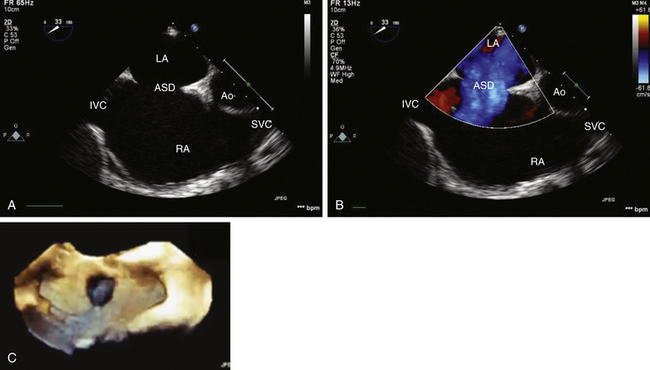
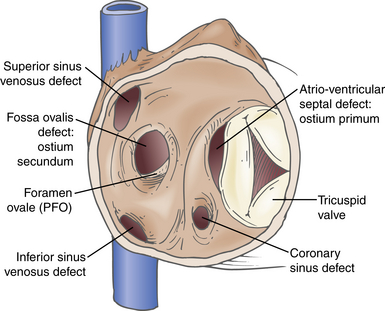
Atrial Septal Defects
General Considerations and Preoperative Evaluation
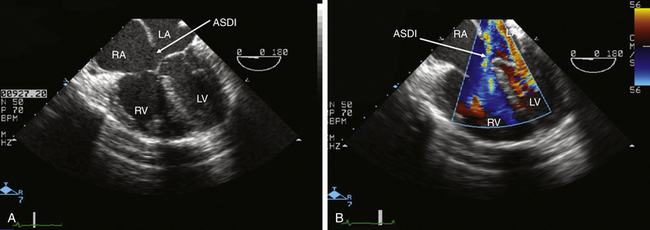
![]() ) and, importantly, with demonstration of shunt flow by color flow Doppler ( Fig. 24-11, B; Video 24-3, B
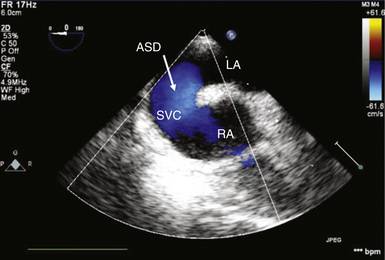
) and, importantly, with demonstration of shunt flow by color flow Doppler ( Fig. 24-11, B; Video 24-3, B ![]() ). Three-dimensional (3D) imaging may also be used to assess the precise localization and size of the defect ( Fig. 24-11, C). Ostium primum and secundum defects are best identified in ME transverse (0 degrees) ( Fig. 24-12) or longitudinal (90 degrees) planes (see Fig. 24-11, A). To delineate the size and tissue rims of these defects, careful assessment in multiple planes, as well as in 3D when available, is necessary. Ostium primum ASD, as a form of AVSD, is usually associated with a cleft in the left-sided AV valve. The sinus venosus defect is best detected in longitudinal planes (90-110 degrees) ( Fig. 24-13) and often requires slight retraction of the ultrasound probe.14,38 In the presence of this defect, careful search for anomalous pulmonary venous drainage using color Doppler is mandatory ( Video 24-4
). Three-dimensional (3D) imaging may also be used to assess the precise localization and size of the defect ( Fig. 24-11, C). Ostium primum and secundum defects are best identified in ME transverse (0 degrees) ( Fig. 24-12) or longitudinal (90 degrees) planes (see Fig. 24-11, A). To delineate the size and tissue rims of these defects, careful assessment in multiple planes, as well as in 3D when available, is necessary. Ostium primum ASD, as a form of AVSD, is usually associated with a cleft in the left-sided AV valve. The sinus venosus defect is best detected in longitudinal planes (90-110 degrees) ( Fig. 24-13) and often requires slight retraction of the ultrasound probe.14,38 In the presence of this defect, careful search for anomalous pulmonary venous drainage using color Doppler is mandatory ( Video 24-4 ![]() ; also see Fig. 24-8). The inferior sinus venosus defect is rare.
; also see Fig. 24-8). The inferior sinus venosus defect is rare.