Acute Pulmonary Embolus
Salil G. Shah
Joshua N. Baker
Thoralf M. Sundt
It is ironic that the heart-lung machine is rarely used today in the treatment of the very condition that inspired its creation: acute pulmonary embolism (PE). Dr. Gibbon was inspired to his life’s work as a research resident at the Massachusetts General Hospital in 1931 as he sat at the bedside of a young woman dying of a massive PE. Have we wrongfully relinquished this part of our surgical heritage?
Acute PE is a remarkably common phenomenon, particularly among hospitalized individuals. The majority of acute pulmonary emboli result in little morbidity, and most are likely entirely unrecognized. Massive PE, however, may lead to hemodynamic collapse and death via a combination of mechanical obstruction of central right ventricular outflow and a peripheral vasospastic response in the remaining unobstructed vasculature. Although such massive emboli represent a relatively small proportion of acute events, they account for significant mortality and morbidity each year by virtue of the frequency with which PE occurs.
Why then is surgical embolectomy an uncommon procedure in most practices? The 2011 American Heart Association Scientific Statement on the management of massive and submassive PE discusses catheter-based embolectomy and thrombolysis more extensively than surgical embolectomy, with the surgical approach being reserved only for patients with contraindication to thrombolysis or in whom thrombolysis has failed. It should be noted that while the guidelines state that “… surgical embolectomy is reasonable for patients… who remain unstable after receiving fibrinolysis,” massive bleeding can be anticipated to complicate any invasive procedure until normal coagulation is restored. The guidelines of the British Thoracic Society published in 2003 are even more dismissive of the role of surgical intervention, with only thrombolysis and “invasive approaches (thrombus fragmentation and IVC filter insertion)” entertained as options for the management of massive PE. Surgical embolectomy, it was noted, was not an option as it was available in few centers.
Why has there been reluctance to offer surgical embolectomy? Apart from logistical issues regarding on-site cardiac surgical support, published mortality rates for acute pulmonary embolectomy traditionally have ranged from 20% to 60%, making it difficult to argue that the surgical results were any better than the natural history. A closer look, however, suggests that the hesitancy to proceed to the operating room may itself be part of the problem. Preoperative cardiac arrest roughly doubles the mortality rate, and the time interval between diagnosis and operation has similarly been shown to have a profound effect on surgical risk. This becomes, of course, a self-perpetuating problem with many physicians, on the basis of the reported high risk of the procedure, reluctant to refer patients for surgery unless profound hemodynamic instability is present. It is only fair to add, however, that technically inadequate surgical embolectomy is likely a contributor to excessive mortality as well.
A critical assessment of the data suggests that there is, in fact, a small but definite place for surgical embolectomy in the management of massive PE. Furthermore, with the remarkable advances in the biocompatibility of extracorporeal membrane oxygenation (ECMO) technology in recent years, the cardiac surgeon, it may be argued, should once again be center stage as part of a multidisciplinary team evaluating all treatment options up front when a patient suffers massive PE. As surgeons we must, of course, take the responsibility for true “around-the-clock” availability and for performing the lowest risk procedure with the best possible results. We aim here to review our current approach to this condition in our hospital including a small number of technical points that are important for both minimizing the intraoperative insult to the struggling right ventricle and optimizing the relief of outflow obstruction. With such an approach, we believe that others can achieve the kind of results reported by Leacche and colleagues in 2006 with a 6% 30-day mortality rate among 47 patients undergoing embolectomy and 1- and 3-year survival rates of 86% and 83%, respectively, or those of Kadner and colleagues with an 8% overall mortality rate including a 28% mortality among patients experiencing a preoperative cardiac arrest.
 DIAGNOSIS
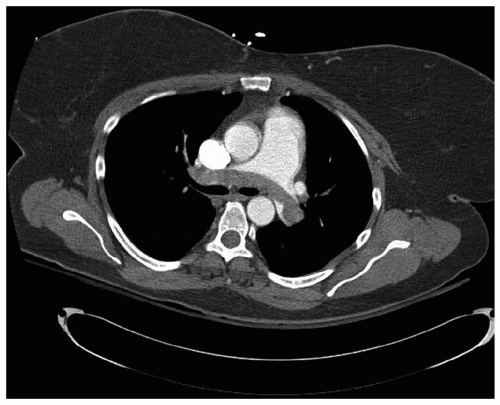
DIAGNOSISIn most instances, the diagnosis of acute PE will have already been made by the time the surgeon is called. Still, it is important to be familiar with current diagnostic modalities, as frequently the patient is being concurrently evaluated for aortic dissection or acute coronary syndrome. Of course, a clinical history consistent with deep vein thrombosis, such as the postoperative orthopedic patient in a long-leg cast, a history of malignancy or pelvic surgical patient after prostatectomy or hysterectomy, or trans-oceaning air travel can be quite helpful. Physical examination is seldom enlightening; however, biomarkers may be revealing; serum D-dimer enzyme-linked immunosorbent assay is almost universally elevated in the presence of acute pulmonary embolus and is frequently used in emergency rooms as a screening test, while elevation in cardiac biomarkers including troponin I and T, and natriuretic peptides have been shown to have negative prognostic impact with the concurrent diagnosis of PE. Nuclear scintigraphy has been almost entirely replaced by helical computerized tomographic (CT) scanning (Fig. 69.1). An adequate scan requires an appropriately timed bolus of contrast material, and a scan performed for other reasons may not image the pulmonary arteries adequately to rule out the diagnosis. Conversely, while CT scanning has the added advantage of providing information about other intrathoracic pathology, a contrast bolus timed appropriately for PE may be inadequate to rule out aortic dissection. The place of nuclear scintigraphy lies principally with
the evaluation of pregnant women and patients allergic to contrast agents or with a marginal renal function.
the evaluation of pregnant women and patients allergic to contrast agents or with a marginal renal function.
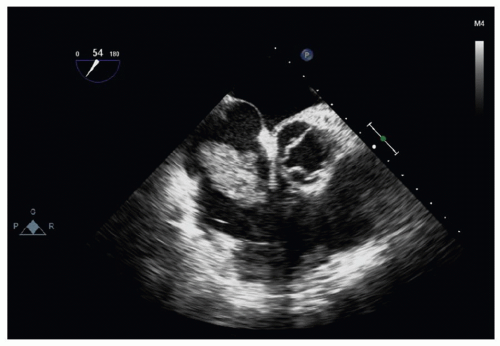
An increasingly important recent diagnostic tool from a surgical standpoint is echocardiography (Fig. 69.2). Right ventricular function can be rapidly assessed in the emergency room or ICU by transthoracic echo, whereas embolic material may be identified in the pulmonary vasculature or, far more importantly, in the atria as “embolus in transit,” including those traversing a patent foramen ovale. Certainly, transesophageal echo should be performed in the operating room if at all possible.
 INDICATIONS FOR SURGERY
INDICATIONS FOR SURGERYWhen called to evaluate the patient at the bedside for surgical intervention, it is important to remember that, in addition to simple mechanical obstruction, the pathophysiology of pulmonary hypertension in acute PE entails the release of serotonin from platelets, histamine from tissues, and circulating thrombin. Hypoxia due to ventilation/perfusion mismatch and increased dead space will also worsen pulmonary vasoconstriction. Accordingly, oxygenation should be optimized before determining that a patient must go to the operating room.
It is widely accepted that systemic hypotension despite inotropic support is an indication for aggressive intervention—surgical, catheter-based, or lytic—as is persistent, refractory hypoxemia. The difficulty, however, is that operative risk is markedly elevated once the patient is in profound shock or suffers cardiac arrest. Accordingly, the challenge is to stratify patients early with regard to risk of poor outcome with anticoagulant therapy alone. In 2005, Sukhija and colleagues showed a reduction in in-hospital mortality among patients with right ventricular dysfunction who received timely pulmonary embolectomy, and multiple other studies have demonstrated the association of right ventricular dysfunction to both short and long-term mortality. Accordingly, the 2011 AHA scientific statement recommends swift intervention either with fibrinolysis or invasive intervention for those with right ventricular dysfunction.
Perhaps because of their wide availability and familiarity with their use in the context of treating acute coronary syndromes and more recently stroke, thrombolytics have taken center stage in the aggressive treatment of the unstable patient with massive PE. It is important to remember, however, that use of thrombolytics is not without risk. In the International Cooperative PE Registry, the incidence of cerebral events with thrombolytics was 3%. Furthermore, Thabut’s meta-analysis of thrombolytic studies reported in 2002 found no improvement in mortality rate when they were used in unselected patients as compared with heparin, but an almost twofold increased risk of hemorrhage (risk ratio 1.76, confidence interval 1.04 to 2.98). To be fair, among the four registries documenting the outcomes of patients with PE (Mappet, ICOPER, PIETE, and EMPEROR), there is suggestion of a decrease in mortality among patients with hemodynamic compromise who are treated with thrombolytics although the numbers are small. There are also a few randomized control trials, mostly in intermediaterisk patients, which show a reduction in pulmonary artery pressures and reduction in thrombus burden albeit with no survival benefit except in high-risk patients.
Catheter embolectomy is another option, and one that is undergoing rapid evolution. Endovascular techniques include clot fragmentation, clot aspiration, and rheolytic therapy. The clinical efficacy of percutaneous therapy alone (Rotares, Aspirex, Angiojet) has been reported to range from 81% to 92% and when combined with thrombolytic therapy to range from 91% to 100% depending on the modality. The mortality rate associated with these interventions, however, has been 16%
to 30%. They are also technically challenging and require a team very familiar with the use of these proprietary devices. While they have gained more widespread acceptance, they are still not universally available. The two most current options are the AngioVac (Vortex Medical Albany, NY), a modified bypass circuit with a movable suction cannula and a series of filters within the circuit that works well for proximal thrombus or embolism, and the EKOS catheter (EKOS Corp, Bothell, WA) that provides for directed thrombolysis and ultrasonic acceleration of clot lysis. Both of these devices show promise, but the data are sparse regarding clinical efficacy currently.
to 30%. They are also technically challenging and require a team very familiar with the use of these proprietary devices. While they have gained more widespread acceptance, they are still not universally available. The two most current options are the AngioVac (Vortex Medical Albany, NY), a modified bypass circuit with a movable suction cannula and a series of filters within the circuit that works well for proximal thrombus or embolism, and the EKOS catheter (EKOS Corp, Bothell, WA) that provides for directed thrombolysis and ultrasonic acceleration of clot lysis. Both of these devices show promise, but the data are sparse regarding clinical efficacy currently.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree