Introduction
The past decade has witnessed major advances in the treatment of atrial fibrillation (AF). Following the seminal observations of Haissaguerre et al1 who recognized the importance of the role of ectopic beats from the pulmonary veins (PVs) in the initiation of AF, percutaneous catheter ablation has evolved as a curative therapy and has been effective in maintaining sinus rhythm in patients with paroxysmal AF. The purpose of this chapter is to discuss the role of ablative therapy and those trials which compare ablation to pharmacologic therapy or to the “ablate and pace” strategy. Because of its necessarily invasive nature, a description is also given of reported complications and studies which have applied strategies to quantify and prevent them.
The main advantage in maintaining sinus rhythm is the relief of symptoms. A significant number of patients with AF complain of palpitations, dizziness, fatigue or dyspnea. Contributory factors include lack of atrial contraction, a rapid ventricular rate, an irregular ventricular rhythm and a reduced cardiac output. In patients with poor ventricular rate control in atrial fibrillation, symptoms of congestive heart failure may result from tachycardia-mediated cardiomyopathy.
In patients with valvular disease (for example, mitral stenosis) or those with a less compliant left ventricle (for example, those with left ventricular hypertrophy), the absence of co-ordinated atrial mechanical activity may result in a reduction in ventricular preload and a consequent reduction in cardiac output. The more rapid heart rates further impair ventricular filling by shortening dia-stolic filling time. Even in patients with controlled ventricular rates, irregular R-R intervals are associated with a 15% reduction in cardiac output.2 Effective rhythm control thus aims to restore normal atrial function, increase ventricular preload and slow the ventricular rate, thereby enhancing cardiac output. These improvements in hemodynamics lead to increased exercise tolerance.3 Other benefits of the maintenance of sinus rhythm include avoidance of tachycardia-induced cardiomyopathy,4 a possible reduction of embolic risk and a potential decrease in mortality.
A few large clinical trials have been published comparing treatment strategies for AF. In particular, the AF Follow-up Investigation of Rhythm Management (AFFIRM),5 Rate Control versus Electrical cardioversion for AF (RACE)6 and Strategies for Treatment of AF (STAF)7 trials compared rate control and rhythm control approaches using antiar-rhythmic drugs (AADs). For a full discussion of these trials see Chapter 35. Adopting an intention-to-treat analysis, these trials concluded that there was no mortality difference between the two approaches. Hence, for the type of patients enrolled, a rate control approach may be adequate treatment for AF when compared with a rhythm control strategy with AADs.
However, it would be incorrect to extrapolate that sinus rhythm offers no benefit over AF. The aforementioned trials were not comparisons of sinus rhythm with AF. They compared a rate control strategy to a pharmacologic rhythm control strategy with AADs which was only poorly effective. When the data in the AFFIRM trial were analyzed according to the patient’ s actual rhythm, the benefit of sinus rhythm over AF became apparent. In a subgroup analysis by Scott et al, the presence of sinus rhythm was one of the most powerful independent predictors of survival, along with the use of warfarin, even after adjustment for all other relevant clinical variables.8 Patients in sinus rhythm were almost half as likely to die compared with those with AF (adjusted hazard ratio 0.53; 99% confidence interval (CI) 0.39 –0.72; P < 0.0001). This benefit was offset apparently by the use of AAD therapy, which may have increased the risk of death. Of note, these trials largely excluded highly symptomatic patients who might benefit most from sinus rhythm.
Inadequacy of non-ablative rhythm control
The failure of AFFIRM, RACE or STAF trials to show any difference between rate and rhythm control strategies is perhaps a testament to the ineffectiveness of the rhythm control strategies that were used. The main modalities for non-ablative rhythm control are AADs and device-based therapy. Meta-analysis of randomized trials looking at the efficacy of AADs showed that up to 32% of the placebo arm patients were in sinus rhythm, compared to 55% of the patients who were assigned to receive ADD therapy.9
Evidently, AADs do not reliably cure AF but serve to reduce the burden of AF in some patients. Even in patients who were able to maintain sinus rhythm while on AADs, debilitating side effects were not uncommon in the published trials. Of the AADs, amiodarone had the highest success rate in maintaining sinus rhythm. However, the side-effect profile of amiodarone led to its discontinuation in up to 30% of patients due to intolerable skin discoloration, pulmonary fibrosis, thyroid dysfunction, neurologic or ophthalmic disorders.10
Device-based therapy has demonstrated poor efficacy for the treatment of AF. Antitachycardia devices can deliver burst atrial pacing and may convert atrial tachycardia or atrial flutter to sinus rhythm but such rapid pacing usually fails to terminate AF.11 Atrial defibrillators can terminate AF with a high success rate.12 However, repeated shocks lead to patient discomfort, making this option intolerable for the majority of patients. Dual site and overdrive atrial pacing,13 which were expected to be beneficial, have failed to demonstrate consistent reduction in AF burden or improvement in AF symptoms.
Another option for patients who have AF with ventricular rates that are uncontrolled despite maximum tolerated AV nodal blocking agents is the so-called “ablate and pace strategy.” The PABA-CHF trial (Pulmonary vein antrum isolation vs AV Node ABlation with Bi-ventricular pacing for Atrial Fibrillation in Congestive Heart Failure), which is currently in press, compared such a strategy to pulmonary vein isolation. This trial is discussed below in the section on randomized controlled trials.
Catheter ablation of atrial fibrillation
Pulmonary vein isolation (PVI), or radiofrequency (RF) ablation for AF directly eliminates or isolates the initiating factors for AF and offers the possibility of a “cure” without subjecting the patient to the side effects of AADs. It is well established that the pulmonary veins play a major role in triggering and maintaining AF. Haissaguerre et al1,14 demonstrated that up to 94% of patients had triggers in one or more pulmonary vein (PV). Sites outside the pulmonary veins may trigger AF in 6 – 10% of patients.15,16
AF has also been reported to be maintained by micro re-entrant circuits (“rotors”) that exhibit high frequency and periodic activity, from which spiral wavefronts of activation radiate into the surrounding atrial tissue.17 Of interest, the dominant rotors in AF are localized primarily in the pulmonary vein-left atrium (PV-LA) region.18 Vagal inputs may also be important in both triggering and maintaining AF; many of these inputs are clustered close to the PV-LA junction.19
The primary goal of radiofrequency catheter ablation for AF is to electrically disconnect the PVs from the rest of the atrium by ablating around the atrial aspect of the veins. Most centers worldwide, regardless of further adjunctive ablation strategies, recognize that PVI is of the foremost importance in AF ablation. The inhomogeneity of left atrial and LA antral anatomy poses significant challenges in PV isolation. The PV is a “funnel-shaped” structure with a large proximal end, known as the antrum. The antrum of each PV blends into the posterior wall of the LA. In order to maximize success and minimize the complication of PV stenosis it is advisable for ablation to be performed around the entire antrum at the posterior left atrial wall.
Most centers performing AF ablation are ablating at the PV antrum and not at the true PV ostium, thus minimizing the risk of PV stenosis. Different centers may refer to ablation at the antrum by various names such as LA catheter ablation, circumferential PV antrum isolation or extraostial isolation. The lesion sets produced by these various approaches are very similar. A typical PV isolation RF ablation lesion set is shown in Plate 38.1.
Randomized controlled trials of catheter ablation for atrial fibrillation
There are five published randomized controlled trials comparing RF ablation with pharmacologic treatment. The sixth published randomized controlled trial compared ablation with no other treatment after patients had undergone prior treatment with amiodarone and direct current (DC) cardioversion. These six published trials are summarized in Table 38.1. Another trial was presented in 2006 but is not yet published. One further trial (in press) compared outcomes in patients with refractory AF who underwent ablation with those who underwent an “ablate and BiV-pace” procedure. At least four further multicenter, randomized trials are in the recruitment stages: AATAC-HF (Ablation v Amiodarone for Treatment of Atrial Fibrillation in patients with Congestive Heart Failure and an implanted ICD/CRTD), CASTLE-AF (Catheter Ablation versus STandard conventional treatment in heart failure patients with Left ventricular dysfunction and Atrial Fibrillation), RAAFT-2 (first line Radiofrequency Ablation versus Antiarrhythmic drugs for Atrial Fibrillation Treatment) and CABANA (Catheter ABlation versus ANtiarrhythmic drug therapy for Atrial Fibrillation – pilot trial).
Table 38.1 Randomized controlled trials of ablation
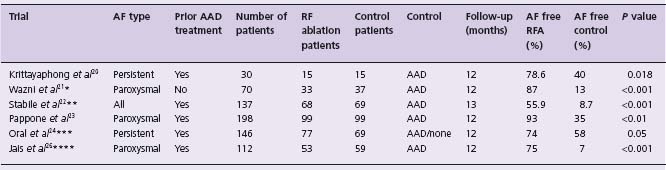
* There were 63 patients with paroxysmal AF and 3 with persistent AF.
* * Patients in the RF group also continued AAD treatment throughout the study.
* * * Crossover to RF from the control arm was permitted after 3 months. There was a high rate of crossover; 77% of patients crossed over by the end of the study. The tabulated results are an intention-to-treat analysis. In the control arm, 3 (4%) patients were in SR in the absence of AAD therapy or RF ablation.
* * * * Crossover to RF from the control arm was permitted after 3 months.
A F, atrial fibrillation; AAD, antiarrhythmic drug (see text for details); R F, radiofrequency.
In a study published in 2003, Krittayaphong et al20 reported on 30 patients with long-standing AF who did not respond satisfactorily to drug treatment. These patients were randomized to treatment with amiodarone (n = 15) or RF ablation (n = 15) and were followed up for 1 year. In the ablation arm, the patients underwent PVI and further ablation in the right atrium. The study reported that freedom from AF was better in the RF ablation group (78.6%) as compared to the amiodarone group (40%) (P = 0.018). There was also a difference in favor of the RF ablation group in terms of subjective measurements of symptoms relating to AF and quality of life. Amiodarone was not observed to have a significant effect on symptoms of AF or quality of life in this study. In the RF ablation group, one patient suffered a stroke. Amiodarone was associated with adverse effects in 47% of patients, necessitating discontinuation in one patient. The authors concluded that RF ablation was an effective alternative treatment in patients with long-standing AF refractory to medical therapy.
Wazni et al21 studied 70 patients (RAAFT pilot study), 67 with paroxysmal and three with persistent AF. None of the included patients had previously been treated for AF; therefore each patient’ s allotted treatment was the first-line treatment for that individual. Patients were randomized to receive either PVI using RF ablation (n = 33) or antiarrhythmic drug treatment (n = 37), with a 1-year follow-up. At the end of 1-year follow-up, 63% of patients who received antiarrhythmic drugs had at least one recurrence of symptomatic AF compared with 13% of patients who received PVI (P < 0.001). There were significantly decreased hospitalization rates and improved quality of life measurements in the PVI group. In the antiarrhythmic drug group, the mean number of AF episodes decreased from 12 to six, after initiating therapy. In the ablation group there was one asymptomatic PV stenosis and no severe PV stenosis. There was no thromboembolic event in either group. There was a higher rate of documented bradycardia in the AAD group, occurring in 9% of patients. Overall, the authors concluded that PVI may be a feasible first-line approach for treating patients with symptomatic AF. This is the only published trial to date comparing outcomes in patients who were AAD na ï ve at the time of recruitment.
Stabile et al22 (CACAF, Catheter Ablation for the Cure of Atrial Fibrillation study) studied 137 patients with both paroxysmal and persistent AF in whom treatment with AAD had already been unsuccessful. Patients were randomized to continue their current drug regimen (n = 69) or to continue medications and additionally undergo RF ablation (n = 68). RF ablation involved PVI, ablation of the cavotricuspid isthmus and ablation from the left inferior pulmonary vein to the mitral valve (mitral isthmus). The follow-up period was 13 months, including a 1-month blanking period. The primary endpoint of the study was the absence of any documented recurrence of atrial arrhythmia lasting >30s in the 1-year follow-up period, following the blanking period. After completion of follow-up, 91% of patients in the non-ablation group had at least one recurrence compared to 44% (P < 0.001) of patients in the ablation group. Of the recurrences in the ablation group, four patients developed an atrial flutter and 26 had recurrence of atrial fibrillation. The success observed in the ablation group must, of course, be viewed in terms of observed complications. In the ablation group there was a major complication rate of 4.4%. Complications were due to a stroke in one patient, a pericardial effusion requiring pericardiocentesis in one patient and transient diaphragmatic paralysis in one further patient. No pulmonary vein stenosis was reported. In the AAD group one patient suffered sudden death, one patient had a transient ischemic attack and two died from cancer. Comparing the median per patient number of hospitalizations in the 1 year of follow-up between the two groups, no statistically significant difference was observed between the ablation and control groups. It was concluded that ablation therapy combined with antiarrhythmic drug therapy is superior to antiarrhythmic drug therapy alone in preventing atrial arrhythmia recurrences in patients with paroxysmal or persistent AF in whom antiarrhythmic drug therapy had already failed.
Pappone et al23 (APAF, Ablation for Paroxysmal Atrial Fibrillation trial) randomized 198 patients with paroxysmal AF who had previously failed AAD therapy. The patients included in this study had a longer history of paroxysmal AF with a mean of 6 ± 5 years. These patients were randomized to circumferential PV ablation (n = 99) or to medical treatment (n = 99) with the maximum tolerated dose of amiodarone, flecainide or sotalol, either as single drug or in combination. Analysis was performed according to the intention-to-treat principle. Of note, 42 of 99 patients who were randomized to drug treatment crossed over and received catheter ablation, which was permitted under the study protocol after 3 months of therapy. All patients received warfarin and were followed for 12 months. In the RF ablation group, a repeat ablation was performed in 9% of patients; this was for atrial tachycardia (3%) and AF (6%). At the completion of follow-up, 86% of patients in the RF ablation group and 22% in the AAD group who did not require a second AAD were free from recurrent AF or atrial tachycardia (P < 0.001). Amiodarone was, overall, administered to 61 patients in the group, either alone or in combination with flecainide. Based on intention-to-treat analysis, after 1 year of follow-up, 93% of the RF ablation group and 35% of the AAD group were free of all atrial tachyarrhythmias (P < 0.01). However, there was a high rate of crossover from the AAD arm to the RF ablation arm, with 42% of patients crossing over to the RF ablation arm. One transient ischemic attack and one pericardial effusion occurred in the RF ablation group. Side effects leading to drug withdrawal were observed in 23 patients in the AAD group; they included thyroid dysfunction (seven patients receiving amiodarone), 1:1 atrial flutter or a wide complex tachycardia (three patients receiving flecainide), and sexual dysfunction (11 patients receiving sotalol). The authors concluded that RF ablation was more successful than AADs for prevention of paroxysmal AF, with an observed low complication rate in the RF ablation group.
Oral et al24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


