The purpose of this meta-analysis was to compare postprocedural mortality and major adverse cardiovascular and cerebrovascular events between transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR) for severe aortic stenosis. Seventeen studies (n = 4,659) comparing TAVI (n = 2,267) and SAVR (n = 2,392) were included. End points were baseline logistic European System for Cardiac Operative Risk Evaluation score, all-cause mortality, cardiovascular mortality, myocardial infarction, stroke, transient ischemic attack, and major bleeding events. Mean differences or risk ratios with 95% confidence intervals were computed, and p values <0.05 were considered significant. The population was matched for risk between the 2 groups on the basis of logistic European System for Cardiac Operative Risk Evaluation score for all outcomes except 30-day all-cause mortality, which had a high-risk population in the TAVI group (p = 0.02). There was no significant difference found in all-cause mortality at 30 days (p = 0.97) and at an average of 85 weeks (p = 0.07). There was no significant difference in cardiovascular mortality (p = 0.54) as well as the incidence of myocardial infarction (p = 0.59), stroke (p = 0.36), and transient ischemic attack (p = 0.85) at averages of 86, 72, 66, and 89 weeks, respectively. Compared with patients who underwent TAVI, those who underwent SAVR had a significantly higher frequency of major bleeding events (p <0.0001) at mean follow-up of 66 weeks. In conclusion, TAVI has similar cardiovascular and all-cause mortality to SAVR at early and long-term follow-up. TAVI is superior to SAVR for major bleeding complications and noninferior to SAVR for postprocedural myocardial infarctions and cerebrovascular events. TAVI is a safe alternative to SAVR in selected high-risk elderly patients with severe aortic stenosis.
Severe aortic stenosis with symptoms or a left ventricular ejection fractions <50% has a poor prognosis and high mortality and is therefore a class I indication for valvular replacement. Surgical aortic valve replacement (SAVR) with suitable implants is a standard of care for such patients and has been shown to alleviate symptoms and to improve survival. Although the standard of care, as many as 1/3 of patients with severe aortic stenosis who require aortic valve replacement are denied SAVR because of associated co-morbidities and poor surgical outcomes. In 2002, transcatheter aortic valve implantation (TAVI) was introduced as an option for this patient group, and it has been shown to reduce mortality and length of hospital stay. The same procedure is also called transcatheter aortic valve replacement to help in uniform communication across the health care community, including patients, researchers, payers, and regulators. The benefits of TAVI in high-risk patients who are not eligible for SAVR are well documented also when compared with medical treatment. However, studies have shown conflicting results for major adverse cardiovascular and cerebrovascular events and mortality after TAVI and SAVR in high-risk patients with aortic stenosis. The purpose of this meta-analysis was to compare the major adverse cardiovascular and cerebrovascular event and mortality outcomes of TAVI and SAVR procedures for severe aortic stenosis.
Methods
We performed this meta-analysis in accordance with the Meta-Analysis of Observational Studies in Epidemiology and Preferred Reporting Items for Systematic Reviews and Meta-Analyses statements for reporting systematic reviews. General guidelines of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.0.2, were used in developing the method, and the meta-analysis was conducted in adherence to these guidelines. We searched the National Library of Medicine’s PubMed database, the National Institutes of Health clinical trials registry, and the Cochrane Central Register of Controlled Trials to recruit clinical studies comparing mortality and major adverse cardiovascular and cerebrovascular events between TAVI and SAVR for severe aortic stenosis. We also searched internet-based sources of information on the results of clinical trials in cardiology ( http://www.cardiosource.com/clinicaltrials , http://www.theheart.org , http://www.clinicaltrialresults.com , and http://www.tctmd.com ), as well as conference proceedings from meetings of the American College of Cardiology, the American Heart Association, the European Society of Cardiology, the Society of Thoracic Surgeons, the Annals of Thoracic Surgery , and Heart Surgery Forum . Searches were restricted to the period from January 2000 through December 2012. The key words used for search were “transcatheter,” “aortic valve,” “aortic stenosis,” “aortic valve replacement,” “TAVI,” and “transcatheter aortic valve replacement (TAVR).” We also reviewed the related links of all relevant reports on PubMed. In addition to our computerized search, we manually reviewed the reference lists of all retrieved reports to complete our search. Two independent investigators (HBP, SD) reviewed all titles and abstracts from the results of our computerized search. The reference list of each report was reviewed manually to complete the search. The selection process is outlined in Figure 1 .
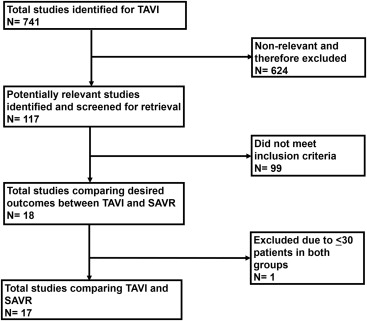
In our analysis, we included clinical studies comparing outcomes between TAVI and SAVR procedures in patients with severe aortic stenosis. To be selected for analysis, a study had to meet all inclusion criteria: (1) the study must compare outcomes of TAVI and SAVR performed in patients with severe aortic stenosis and (2) the study must report ≥1 of the following outcomes: all-cause mortality, cardiovascular mortality, myocardial infarction (MI), stroke, transient ischemic attack (TIA), and major bleeding events. Studies with ≤30 patients in either group were excluded. Studies that did not meet any of the inclusion criteria were excluded.
After identifying all relevant reports, we extracted characteristics of each study (investigators, year, design, sample size, follow-up duration, type of valve placed, type of TAVI approach, baseline clinical characteristics of the patient population, and baseline logistic European System for Cardiac Operative Risk Evaluation (EuroSCORE) score, representing preoperative risk) and postprocedural outcomes measured. End points extracted were logistic EuroSCORE, all-cause mortality (30 days and long term), cardiovascular mortality, MI, stroke, TIA, and major bleeding events. The target of the study was to evaluate mortality and major adverse cardiovascular and cerebrovascular events. Two reviewers (HBP, SD) independently extracted data and assessed outcomes. Interrater agreement was 90%, and disagreements were resolved by consensus.
Meta-analysis was performed according to the recommendations of the Cochrane Collaboration and in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. The combined risk ratio (RR) or mean difference across all studies with corresponding 95% confidence interval (CI) was calculated for each end point using RevMan version 5.1 statistical software for dichotomous and continuous outcomes, respectively (The Cochrane Collaboration, Copenhagen, Denmark). Heterogeneity of the studies was assessed for each end point. Those studies that were homogeneous for an end point were analyzed using the Mantel-Haenszel fixed-effect model, and those studies that were heterogeneous for an end point were analyzed using the random-effect model. A 2-sided α error of <0.05 was considered to be statistically significant.
Results
The search process is explained in Figure 1 . Only 17 studies met the inclusion criteria and were included in our analysis. Overview and baseline patient characteristics are listed in Table 1 . Smith et al published 1-year outcomes and Kodali et al published 2-year outcomes of same patients from the Placement of Aortic Transcatheter Valves (PARTNER) trial. Ten studies had matched propensity groups between TAVI and SAVR, whereas in 6 studies, TAVI was performed in high-risk patients compared with patients in SAVR group. Higgins et al did not compare baseline logistic EuroSCORE between 2 groups. The average per patient follow-up for each outcome is listed in Table 2 .
| Study | Design | Patient Characteristics | Total Population | Valve Used | TAVI Approach | Mean Follow-Up (days) | End Points | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SAVR | TAVI | SAVR | TAVI | SAVR | TAVI | |||||||
| B | M | TF | TA | |||||||||
| Walther et al (2010) | Retrospective | Age 70.3 ± 9.9 yrs, LES 30 ± 13 | Age 70.3 ± 9.9 yrs, LES 29 ± 13 | 100 | 100 | — | — | ES | 0 | 100 | 365 | +, long-term all-cause mortality, stroke |
| Clavel et al (2010) | Retrospective | Age 70.0 ± 10 yrs, female 19%, LES 18 ± 14, CAD 66% | Age 81 ± 8 yrs, female 41%, LES 32 ± 18, CAD 79% | 200 | 83 | 142 | 58 | Cribier-Edwards/ES | 44 | 39 | 365 | + |
| Wenaweser et al (2011) | Prospective | Age 70.3 ± 9.9 yrs, female 49.5%, LES 12.5 ± 8.2, CAD 52.3% | Age 70.3 ± 9.9 yrs, female 56%, LES 24.7 ± 24.9, CAD 65.0% | 107 | 257 | 107 | 0 | CV and ES | — | — | 900 | +, long-term all-cause mortality, CVM, stroke, TIA, MI |
| Smith et al (2011) | RCT | Age 84.5 ± 6.4 yrs, female 42.2%, LES 29.2 ± 15.6, CAD 76.9% | Age 83.6 ± 6.8 yrs, female 43.3%, LES 29.3 ± 16.5, CAD 74.9% | 351 | 348 | — | — | ES | 244 | 104 | 365 | +, long-term all-cause mortality, CVM, stroke, MI |
| Higgins et al (2011) | Retrospective analysis of prospective registry | Age 78 yrs, female 59% | Age 78 yrs, female 47% | 46 | 46 | — | — | — | 0 | 46 | 30 | +, stroke |
| Johansson et al (2011) | Prospective | Age 81 ± 5 yrs, female 50%, LES 23 ± 14, CAD 57.5% | Age 81 ± 6 yrs, female 50%, LES 24 ± 17, CAD 62.5% | 40 | 40 | — | — | ES | 10 | 30 | 730 | +, long-term all-cause mortality |
| Stohr et al (2011) | Retrospective | Age 79.3 ± 3.3 yrs, female 55.9%, LES 16.7 ± 9.1, CAD 58% | Age 80.2 ± 6.4 yrs, female 63.7%, LES 21.2 ± 13.1, CAD 62% | 175 | 175 | — | — | ES 52%, CV 48% | 73 | 82 | 30 | +, stoke |
| Conradi et al (2012) | Prospective | Age 82.5 ± 4.1 yrs, female 58.5%, LES 23.6 ± 10.4, CAD 42.7% | Age 81.9 ± 5.2 yrs, female 63.4%, LES 23.9 ± 11.5, CAD 51.2% | 82 | 82 | — | — | ES | 22 | 60 | 180 | +, long-term all-cause mortality, stroke |
| Appel et al (2012) | Prospective | Age 77 ± 5 yrs, female 51%, LES 8 ± 4, CAD 9% | Age 81 ± 8 yrs, female 51%, LES 16 ± 11, CAD 29% | 45 | 45 | — | — | ES | 29 | 16 | 180 | +, long-term all-cause mortality, MI, stroke |
| D’Errigo et al (2012) | Prospective cohort study | Age 78.6 ± 6.9 yrs, female 39.9%, LES 9.4 ± 10.4, CAD 26.2% | Age 79.4 ± 7.4 yrs, female 37.6%, LES 8.8 ± 9.5, CAD 29 | 133 | 133 | — | — | ES-XT and CV | — | — | 30 | +, stroke, MI |
| Holzhey et al (2012) | Retrospective | Age 80.5 ± 4.6 yrs, female 64.7%, LES 18.3 ± 14 | Age 79.8 ± 5.4 yrs, female 64.7%, LES 18.7 ± 2.7 | 167 | 167 | — | — | — | 0 | 167 | 657 | +, stroke, TIA |
| Nielsen et al (2012) | RCT | Age 82 ± 4.4 yrs, female 66.6%, LES 10.3 ± 5.8 | Age 80 ± 3.6 yrs, female 73.5%, LES 9.4 ± 3.9 | 36 | 34 | — | 36 | ES | 0 | 34 | 90 | +, long-term all-cause mortality, MI, stroke, TIA, major bleeding |
| Motloch et al (2012) | Retrospective | Age 76 ± 0.5 yrs, female 38.4%, LES 10.7 ± 1, CAD 41.9% | Age 81 ± 0.7 yrs, female 56%, LES 27.4 ± 1.8, CAD 48.8% | 86 | 84 | — | — | ES | 41 | 43 | 3 | Stroke, TIA, major bleeding |
| Tamburino et al (2012) | Retrospective | Age 70.3 ± 9.9 yrs, female 51.2%, LES 6.8 ± 5.9, CAD 8.0% | Age 80.9 ± 5.2 yrs, female 53.7%, LES 21.1 ± 14.2, CAD 19.3% | 400 | 218 | — | — | CV 89%, ES 11% | 214 | 0 | 365 | +, long-term all-cause mortality, CVM, stroke, MI, major bleeding |
| Miller et al (2012) | RCT | Age 84.4 ± 6.3 yrs, female 42.8%, LES 29.2 ± 15.2, CAD 77.7% | Age 83.6 ± 6.8 yrs, female 42.8%, LES 29.4 ± 16.5, CAD 75.2% | 313 | 344 | — | — | ES | 240 | 104 | 730 | +, long-term all-cause mortality, stroke, TIA |
| Latib et al (2012) , ∗ | Retrospective | Age 79.4 ± 3.0 yrs, LES 24.4 ± 13.4, CAD 45.9% | Age 80.5 ± 6.9 yrs, LES 23.2 ± 15.1, CAD 39.6% | 111 | 111 | — | — | ES/ES-XT 58.3%, CV 41.7% | 111 | 0 | 365 | +, long-term all-cause mortality, CVM, MI, stroke, TIA, major bleeding |
| Kodali et al (2012) | RCT | Age 84.5 ± 6.4 yrs, female 42.2%, LES 29.2 ± 15.6, CAD 76.9% | Age 83.6 ± 6.8 yrs, female 43.3%, LES 29.3 ± 16.5, CAD 74.9% | 351 | 348 | — | — | ES | 244 | 104 | 730 | +, long-term all-cause mortality, CVM, stroke, MI |
| Outcome | Study | Total Population | Follow-Up (days) | Mean per Patient Follow-Up (days) |
|---|---|---|---|---|
| MI | Appel et al (2012) | 90 | 180 | 504 |
| Tamburino et al (2012) | 618 | 365 | ||
| Nielsen et al (2012) | 70 | 90 | ||
| D’Errigo et al (2012) | 266 | 30 | ||
| Kodali et al (2012) (PARTNER-2) | 699 | 730 | ||
| Latib et al (2012) | 222 | 365 | ||
| Wenaweser et al (2011) | 364 | 900 | ||
| Stroke | Holzhey et al (2012) | 334 | 657 | 463 |
| Motloch et al (2012) | 170 | 3 | ||
| Stohr et al (2011) | 350 | 30 | ||
| Conradi et al (2012) | 164 | 180 | ||
| Appel et al (2012) | 90 | 180 | ||
| Tamburino et al (2012) | 618 | 365 | ||
| Miller et al (2012) | 657 | 730 | ||
| Nielsen et al (2012) | 70 | 90 | ||
| D’Errigo et al (2012) | 266 | 30 | ||
| Higgins et al (2011) | 92 | 30 | ||
| Kodali et al (2012) (PARTNER-2) | 699 | 730 | ||
| Latib et al (2012) | 222 | 365 | ||
| Walther et al (2010) | 200 | 365 | ||
| Wenaweser et al (2011) | 364 | 900 | ||
| TIA | Latib et al (2012) | 222 | 365 | 625 |
| Walther et al (2010) | 200 | 365 | ||
| Wenaweser et al (2011) | 364 | 900 | ||
| Holzhey et al (2012) | 334 | 657 | ||
| Motloch et al (2012) | 170 | 3 | ||
| Miller et al (2012) | 657 | 730 | ||
| Nielsen et al (2012) | 70 | 90 | ||
| Kodali et al (2012) (PARTNER-2) | 699 | 730 | ||
| Long-term all-cause mortality | Appel et al (2012) | 90 | 180 | 593 |
| Tamburino et al (2012) | 618 | 365 | ||
| Miller et al (2012) | 657 | 730 | ||
| Nielsen et al (2012) | 70 | 90 | ||
| Johansson et al (2011) | 80 | 730 | ||
| Kodali et al (2012) | 699 | 730 | ||
| Latib et al (2012) | 222 | 365 | ||
| Walther et al (2010) | 200 | 365 | ||
| Wenaweser et al (2011) | 364 | 900 | ||
| Major bleeding | Motloch et al (2012) | 170 | 3 | 463 |
| Tamburino et al (2012) | 618 | 365 | ||
| Nielsen et al (2012) | 70 | 90 | ||
| Kodali et al (2012) (PARTNER-2) | 699 | 730 | ||
| Latib et al (2012) | 222 | 365 | ||
| Cardiovascular mortality | Kodali et al (2012) (PARTNER-2) | 699 | 730 | 601 |
| Latib et al (2012) | 222 | 365 | ||
| Tamburino et al (2012) | 618 | 365 | ||
| Wenaweser et al (2011) | 364 | 900 |
A total of 17 studies compared 4,659 patients with severe aortic stenosis who underwent TAVI (n = 2,267) and SAVR (n = 2,392). The mean logistic EuroSCORE comparison for each outcome is listed in Table 3 . Thirty-day all-cause mortality was not significantly different between the TAVI and SAVR groups (RR 1.00, 95% CI 0.8 to 1.23, p = 0.97; Figure 2 ). The subgroup analysis of randomized controlled trials also did not show any significant difference in 30-day all-cause mortality between the TAVI and SAVR groups (RR 1.53, 95% CI 0.99 to 2.36, p = 0.06; Figure 2 ). Long-term all-cause mortality was not significantly different between the TAVI and SAVR groups (RR 0.78, 95% CI 0.59 to 1.02, p = 0.07) at 85 weeks of mean follow-up ( Figure 3 ). The subgroup analysis of randomized controlled trials also did not show any significant difference in long-term mortality between the TAVI and SAVR groups (RR 0.94, 95% CI 0.8 to 1.1, p = 0.41) at 99 weeks of mean follow-up ( Figure 3 ). Cardiovascular mortality was not significantly different between the TAVI and SAVR groups (RR 0.92, 95% CI 0.71 to 1.19, p = 0.54) at mean follow-up of 86 weeks ( Figure 4 ). The incidence of MI was not significantly different between the TAVI and SAVR groups (RR 1.28, 95% CI 0.52 to 3.17, p = 0.59) at mean follow-up of 72 weeks ( Figure 5 ). The subgroup analysis of randomized controlled trials also did not show any significant difference in the incidence of MI between the TAVI and SAVR groups (RR 2.43, 95% CI 0.48 to 12.29, p = 0.28) at mean follow-up of 104 weeks ( Figure 5 ).
| End Point | Overall LES | RCT LES | ||||
|---|---|---|---|---|---|---|
| MD | 95% CI | p Value | MD | 95% CI | p Value | |
| 30 day all-cause mortality | −3.66 | −6.86 to −0.46 | 0.02 | 0.22 | −1.15 to 1.59 | 0.75 |
| Long-term all-cause mortality | −3.7 | −8.35 to 0.95 | 0.12 | 0.22 | −1.15 to 1.59 | 0.75 |
| Cardiovascular mortality | −6.39 | −14.67 to 1.89 | 0.13 | — | — | — |
| MI | −4.56 | −10.01 to 0.9 | 0.1 | 0.42 | −1.24 to 2.07 | 0.62 |
| Stroke | −4.1 | −9.43 to 1.23 | 0.13 | 0.22 | −1.15 to 1.59 | 0.75 |
| TIA | −3.34 | −11.05 to 4.38 | 0.4 | 0.22 | −1.15 to 1.59 | 0.75 |
| Major bleeding | −5.87 | −14.39 to 2.64 | 0.18 | 0.42 | −1.24 to 2.07 | 0.62 |

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


