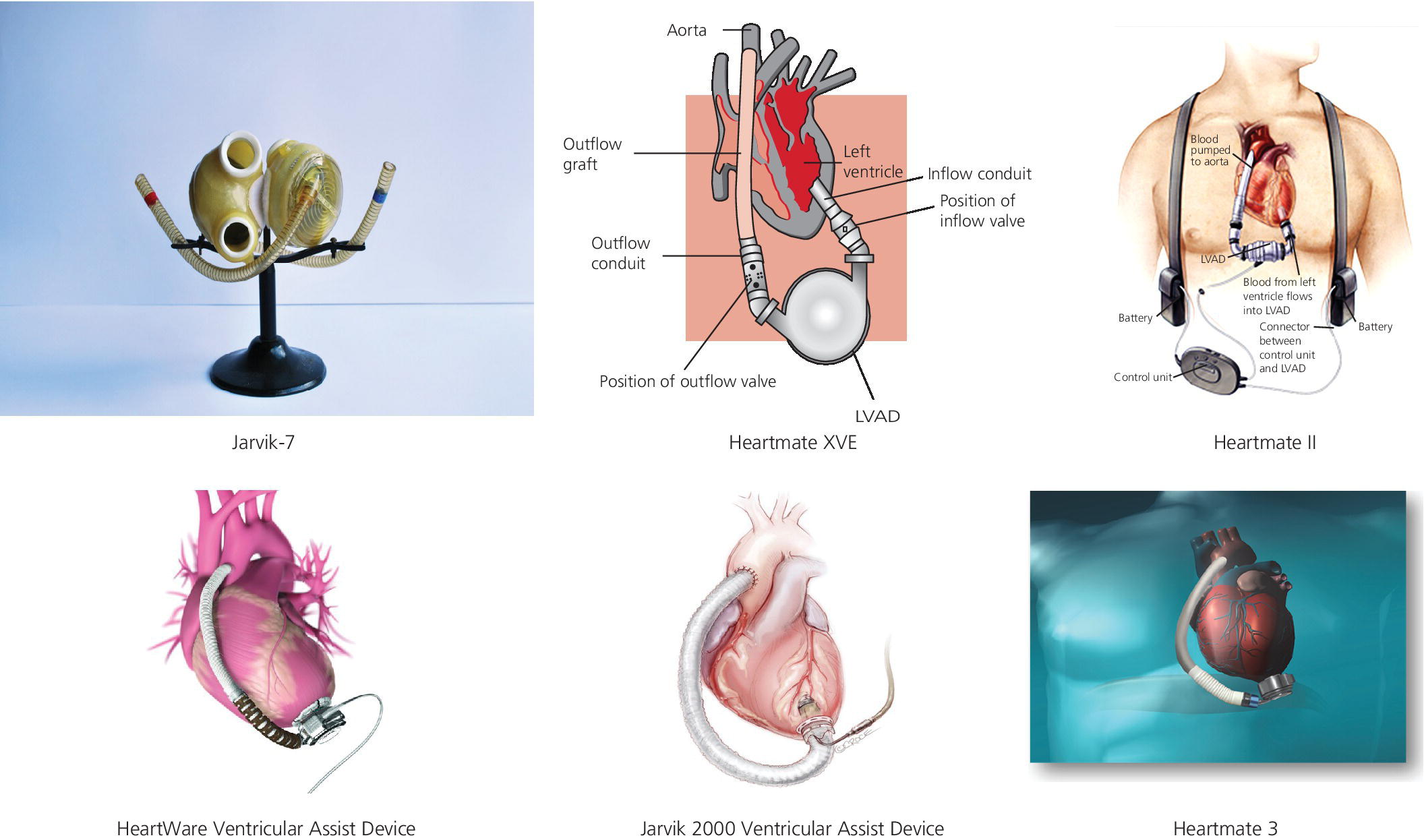
CHAPTER 22 Brett C. Sheridan and Jason N. Katz The modern history of left ventricular assist devices (LVAD) dates back to the late 1970s and early 1980s with the development of the Jarvik artificial heart. In 1982, surgeons at the University of Utah implanted the Jarvik 7 prototype (Figure 22.1) in a patient named Barney Clark, who ultimately survived 112 days on mechanical support [1]. At the same time, Dr. Norman Shumway and his group at Stanford University were improving the surgical technique for cardiac transplantation, and found durable success thanks in large part to the pharmacologic discovery of inducible immune tolerance. Research and development resources were concentrated on improving on the early promise of heart transplantation as the primary strategy for treating end‐stage heart failure, given its biologic symmetry compared to the Frankensteinian mechanical support alternatives. This slowed the evolution of mechanical support technologies throughout the 1980s and into the 1990s. Figure 22.1 Different types of mechanical cardiac support devices. The conventional wisdom at the time was that heart replacement therapy would require two pumps to support both the left and right ventricular chambers, and this concept created additional barriers to the maturation of mechanical circulatory support (MCS) as a field. A turning point came in 2001 with the publication of the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) trial [2]. This pivotal study was the first to demonstrate a durable advantage of LVAD therapy for transplant‐ineligible patients with advanced heart failure. With a pneumatically driven pulsatile left ventricular support system (Figure 22.1; HeartMate XVE, Thoratec Corp., Pleasanton, CA, USA), investigators showed a nearly 50% reduction in all‐cause mortality compared to optimal medical management alone. These results validated two critical concepts: (a) that univentricular (i.e., left‐sided) support could work in advanced, biventricular heart failure; and (b) that MCS could improve outcomes versus medical therapy for individuals with extremely advanced heart failure. Although the utilization of LVAD therapy slowly increased following REMATCH, it became apparent that the early pulsatile devices (e.g., HeartMate XVE; Novacor, World Heart Corp., CA, USA) were imperfect solutions to the heart failure epidemic. Limitations of these first‐generation LVAD included their large size and limited durability. Many of these barriers were addressed with the creation of second‐generation, axial‐flow pumps (Figure 22.1; HeartMate II, Thoratec Corp., CA, USA). Results from a multicenter trial of 133 nonrandomized patients receiving the HeartMate II device as a bridge to transplantation, published in 2007 [3], demonstrated that patients could be successfully stabilized until cardiac transplantation with the use of a continuous‐flow LVAD. In addition, the HeartMate II device was found to be easier to implant, more durable, and had fewer complications that its pulsatile predecessors. The HeartMate II was also tested as an alternative to the first‐generation HeartMate XVE in a transplant‐ineligible heart failure population. Although both devices improved quality of life, the axial‐flow HeartMate II device allowed for greater survival free from stroke, with less risk of device failure [4]. With validation of LVAD therapy as both a bridge to transplantation and a destination‐therapy option, other industry participants began to enter the MCS space. Currently, the HeartWare ventricular assist device (HVAD) and Jarvik 2000 ventricular assist device are being studied in a variety of heart failure groups, while the St. Jude Corporation has embarked on a randomized controlled trial of its third‐generation, magnetically levitated, centrifugal‐flow HeartMate 3 device (Figure 22.1). Predictably, the utilization of LVAD has increased at a remarkable rate since about 2008, and continues to accelerate thanks in large part to rapidly evolving technology, increasing operator experience (with improved outcomes), decreasing donor organ availability, and a burgeoning population of aging heart failure patients. To better understand patient eligibility, appropriate timing of implantation, and the management of LVAD‐supported individuals in both the acute and chronic post‐operative settings, it is critical to understand their hemodynamic profiles. There are multiple factors that should be considered when assessing a patient’s eligibility for LVAD. It is critical to evaluate not only their expected tolerability of the initial operative insult, but also the likelihood that the patient will reap durable benefits from mechanical support. Those individuals most likely to thrive with univentricular support include those with pre‐operative hemodynamics most suggestive of pure left‐sided cardiac dysfunction. On the other hand, individuals with isolated right‐sided heart failure, or those with biventricular failure in whom right ventricular dysfunction is not modifiable, are poor candidates for LVAD therapy and should not undergo device implantation. The following are some hemodynamic examples to guide operative assessment. The ideal LVAD patient has predominantly advanced left ventricular dysfunction. These individuals will have elevated left‐sided cardiac filling pressures, and often secondarily increased right‐sided filling pressures (Table 22.1). As stroke volume diminishes, mean systemic perfusion (mean arterial pressure, MAP) will decrease as well. In addition, mixed venous oxygen saturation (SVO2) will be low. If, on the other hand, left‐sided filling pressures are not found to be elevated, it can be surmised that the LVAD will be unlikely to provide incremental left ventricular unloading, and hence device implantation would result in little benefit at the expense of increased complication.
Hemodynamics of left ventricular assist device implantation
Initial evaluation
Left‐sided heart failure
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


