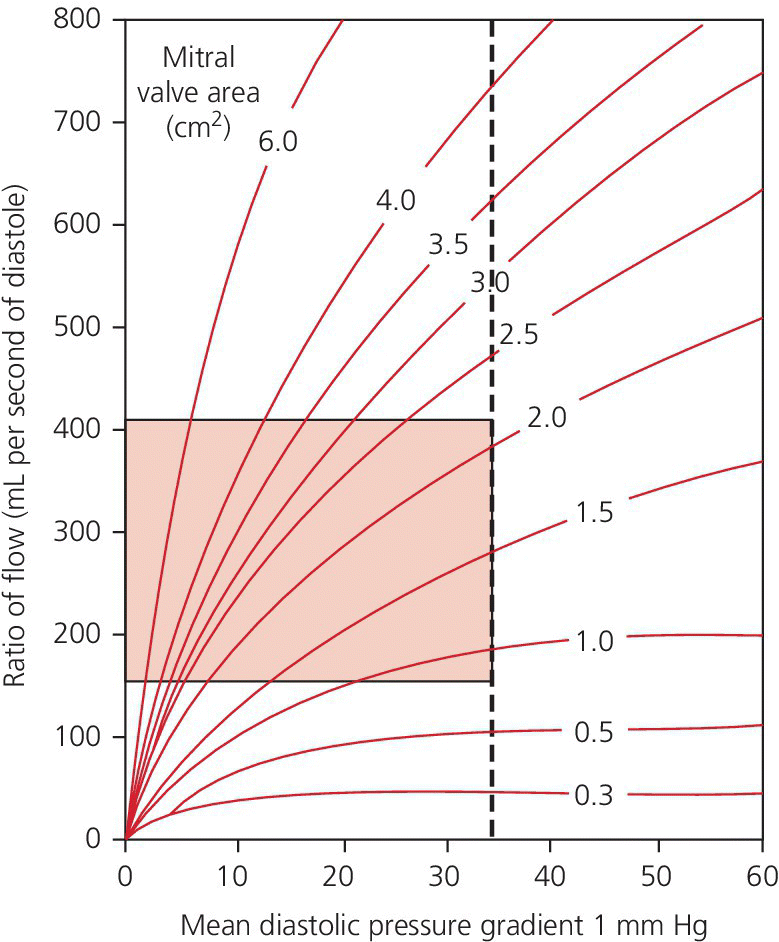
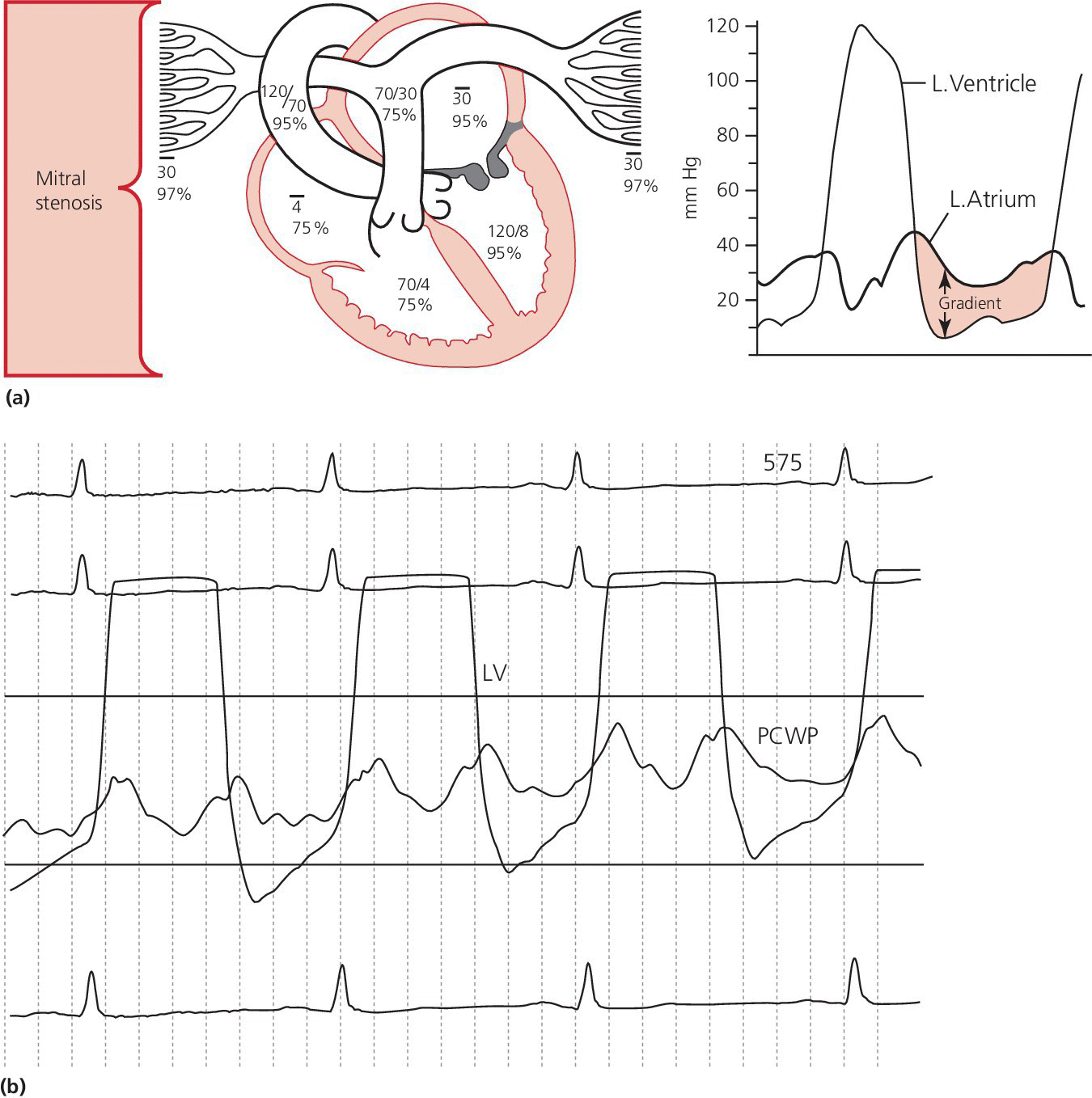
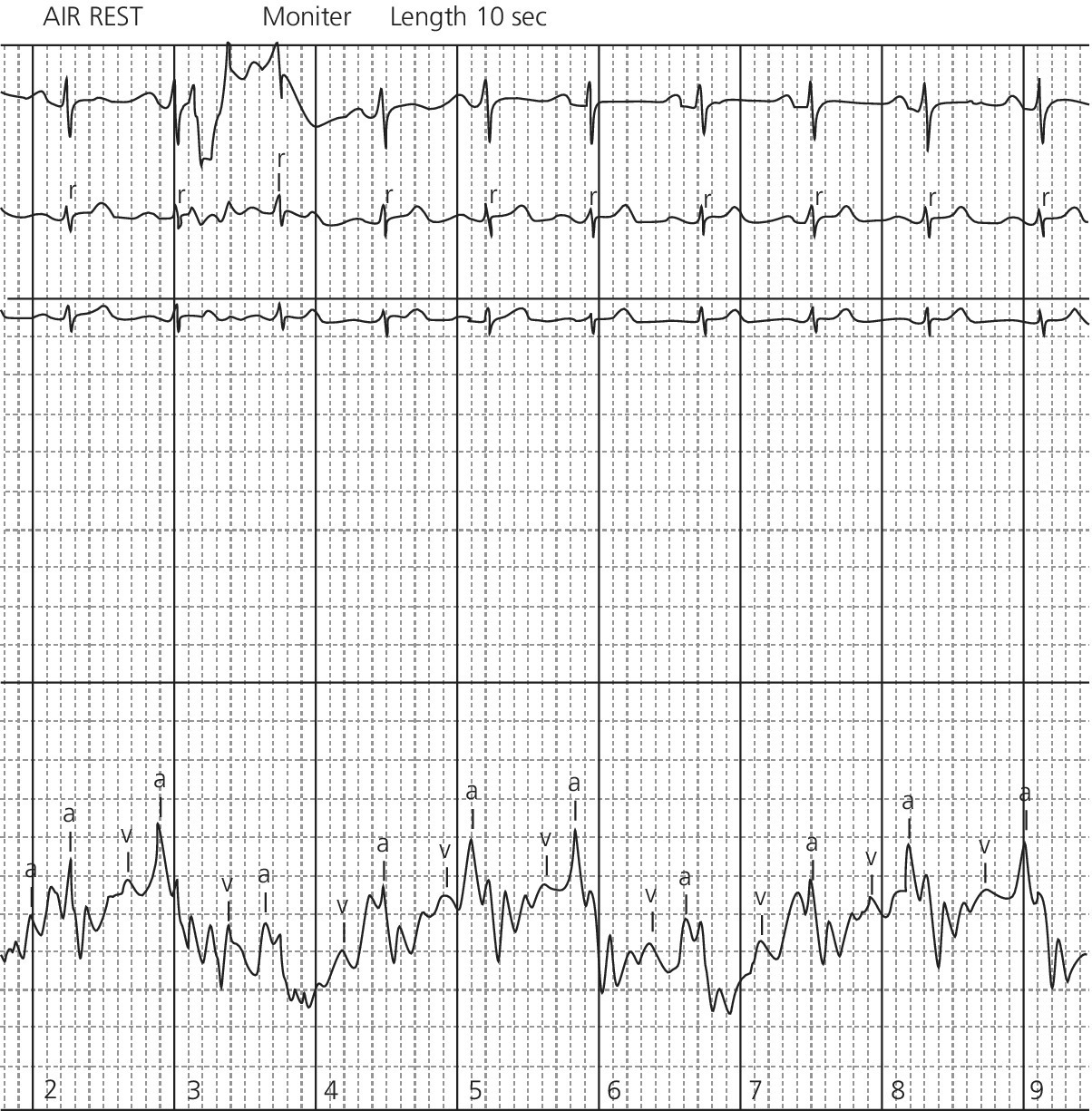
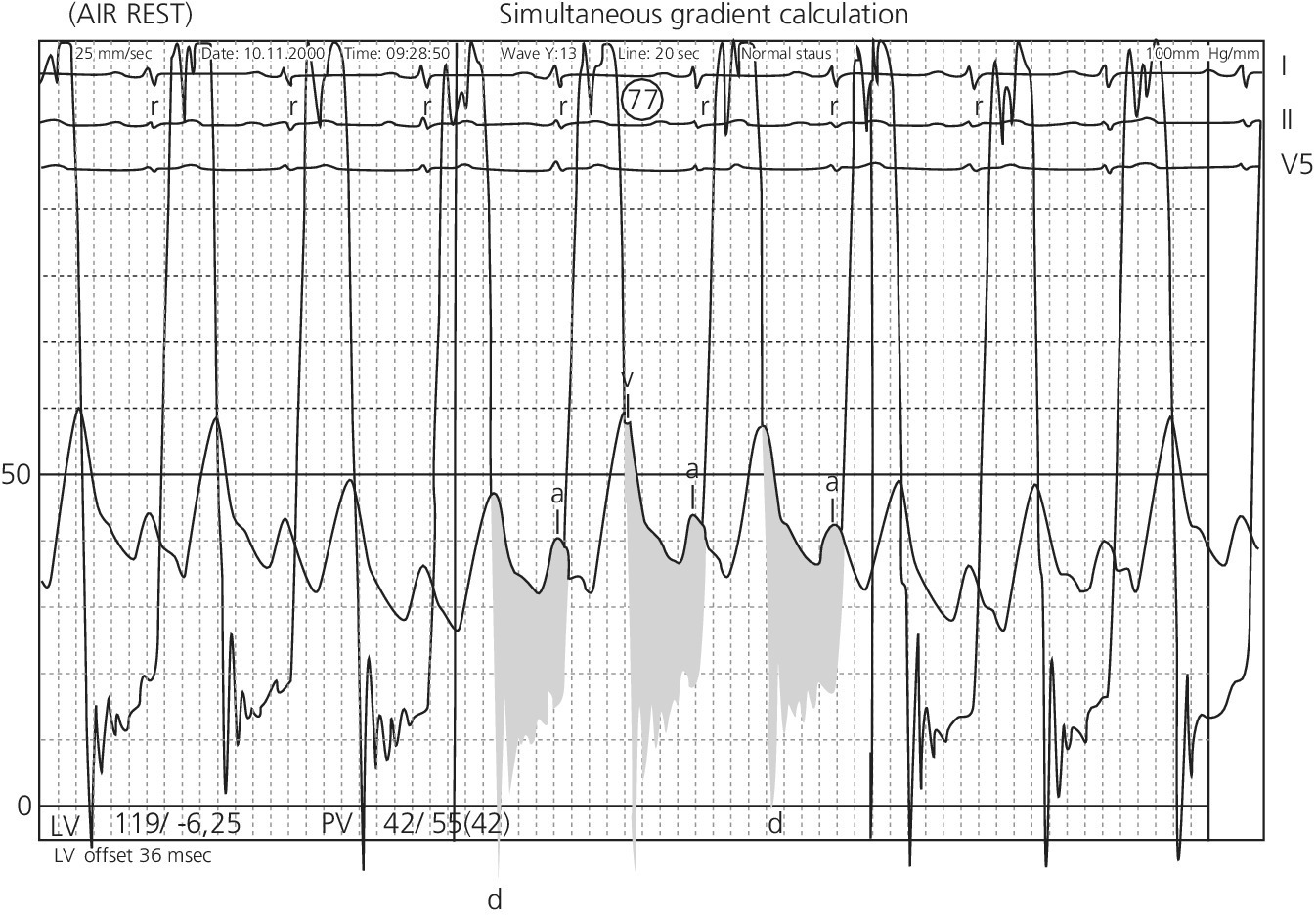
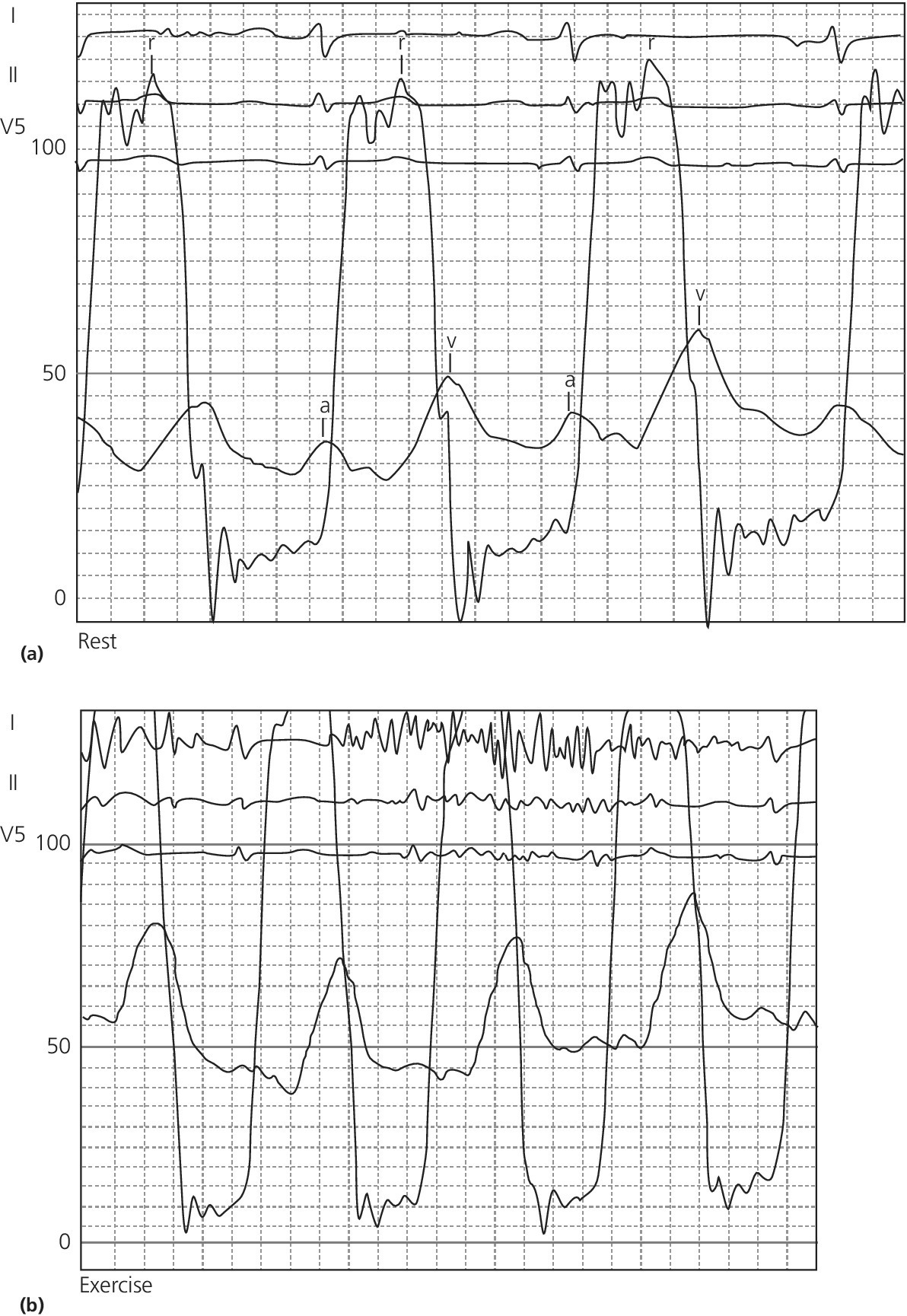
CHAPTER 10 Robert V. Kelly, Chadwick Huggins and George A. Stouffer The cross‐sectional area of the mitral valve is 4–6 cm2 in healthy adults. Mitral stenosis (MS) occurs when this area decreases, resulting in increased resistance to flow from the left atrium (LA) to left ventricle (LV), resulting in increased LA pressure. The increased LA pressure is transmitted to the pulmonary circulation, resulting in elevated right heart pressures and in some cases elevated pulmonary vascular resistance (Figure 10.1). Figure 10.1 Hemodynamic changes of mitral stenosis. [LA = left atrium; LV = left ventricle; PA = pulmonary artery; PV = pulmonary veins; RA = right atrium; RV = right ventricle.] Almost all cases of MS are rheumatic in origin. Isolated MS occurs in 40% of all adults presenting with rheumatic heart disease and women with MS outnumber men by approximately 2:1. Rheumatic mitral valve changes including thickening of the mitral valve leaflets, calcification, and commissural fusion, which occurs over the course of decades. Other rare causes of MS include systemic lupus erythematosis, rheumatoid arthritis, carcinoid, mucopolysaccharidosis, mitral annular calcification, and congenital valve deformity. There are some rare conditions that cause increased LA pressure that can mimic MS. Examples include LA myxoma, pulmonary vein obstruction, and cor triatriatum (a thin membrane across the left atrium that obstructs pulmonary venous inflow). MS is a progressive disorder with symptoms worsening as mitral valve area decreases. Symptoms do not usually appear until the mitral valve area is <2 cm2. Initially, symptoms occur only with exertion, emotional stress, or pregnancy and consist primarily of dyspnea. Symptoms can progress over time and in severe cases eventually include dyspnea at rest, orthopnea, and paroxysmal nocturnal dyspnea. More rarely, there may be angina, palpitations, recumbent cough, and/or hemoptysis. Symptoms can occur more acutely if the patient becomes pregnant, develops a systemic illness, or goes into atrial fibrillation. Patients with MS generally pass through four separate hemodynamic stages (Figure 10.2): Figure 10.2 Hemodynamic effects of different degrees of mitral stenosis (calculations performed assuming HR × DFP = 32). In addition to increasing resistance to flow, MS also increases transvalvular blood velocity and the ratio of turbulent to laminar flow across the mitral valve. For example, LV inflow velocity across the normal valve is approximately 1 m/s, but can reach 3 m/s in severe MS. The effects of accelerated velocity of blood are not completely understood. The classic hemodynamic findings associated with MS include elevated LA pressure and a gradient between the LA and LV that persists throughout diastole (Table 10.1). There are several other findings that are discussed in the following in relationship to the cardiac chamber involved. Table 10.1 Findings at cardiac catheterization. Figure 10.3 Transvalvular pressure gradient in a patient with MS. Schematic (a) and actual recordings (b) of simultaneous LV and PCWP pressures in a patient with MS. Note that in (b) the PCWP is delayed relative to LV and should be phase shifted prior to performing any calculations. Figure 10.4 RA tracing from a patient with severe MS. Note the prominent A wave. In mild MS, pulmonary artery pressures may be normal or slightly elevated at rest but increase with exercise. In severe MS, pulmonary artery pressure will be elevated at rest. Pulmonary hypertension in severe MS can be caused by both elevated LA pressures and increased pulmonary vascular resistance due to pulmonary arteriolar constriction and obliterative changes in the pulmonary vascular bed. It has been speculated that the increased resistance in the pulmonary veins protects the patient from pulmonary edema. Evidence for a reversible component of pulmonary vascular resistance was shown in a study of 18 women with MS and pulmonary hypertension. Inhaled nitric oxide reduced pulmonary artery systolic pressure by approximately 15% and pulmonary vascular resistance by 30% without having any effect on LV end‐diastolic pressure, LA pressure, cardiac output, or systemic vascular resistance [2]. Following mitral valve surgery or percutaneous balloon mitral valvuloplasty (PBMV), pulmonary artery pressures decrease immediately due to decreased LA pressure. Over time, pulmonary artery pressures continue to decrease as pulmonary vascular resistance decreases. This was demonstrated in a study of 21 patients with severe MS and pulmonary hypertension undergoing PBMV. Mean PCWP decreased from 27 ± 5 (pre‐procedure) to 15 ± 4 mm Hg (immediately post‐procedure) and then remained constant at a mean follow‐up of 1 year. Mean mitral valve gradient decreased from 18 ± 4 to 6 ± 2 mm Hg and then remained constant. Pulmonary artery systolic pressure decreased from 65 ± 13 to 50 ± 13 mm Hg to 38 ± 9 mm Hg. Pulmonary vascular resistance decreased from 461 ± 149 to 401 ± 227 to 212 ± 99 (dyn s/cm5) [3]. In most patients with MS, the LV end‐diastolic pressure (LVEDP) is normal. It is reduced in about 15% of cases. In the presence of heart failure or mitral regurgitation (MR), LVEDP can be elevated. Since flow across the mitral valve in MS is directly proportional to transvalvular pressure gradient, as LVEDP increases LA pressure must also increase to maintain the transvalvular pressure gradient. Also, reduced LV compliance from (a) displacement of the interventricular septum secondary to early filling of the right ventricle or (b) interstitial fibrosis from rheumatic fever may hinder LV filling during the second half of diastole. Right ventricular systolic pressures rise in line with the increases in pulmonary artery systolic pressures. The severity of MS can be assessed by cardiac catheterization. The mitral valve gradient may be directly measured by comparing simultaneous pressures in the LV and LA (either by directly measuring LA pressures or using PCWP; Figure 10.5). Simultaneous LA and LV pressures are recorded and the gradients measured. For best results, the fastest paper recording speed (generally 100 mm/s) and a 0–50 mm Hg scale is used. The gradient across the mitral valve should be measured using an average of five cardiac cycles in patients with normal sinus rhythm and ten cardiac cycles in patients with atrial fibrillation. Figure 10.5 Transvalvular gradient in a patient with MS. Simultaneous recording of LV and PCWP pressure tracings. The darkened portion represents transvalvular gradient. The severity of obstruction in MS can be quantified using mitral valve area, mitral valve resistance, or mitral valve gradient. Mitral valve area remains the standard used in most laboratories because it includes the three major hemodynamic variables: transvalvular pressure gradient, cardiac output, and diastolic filling period. By convention, a mitral valve area <1 cm2 is considered severe MS, a valve area of 1–1.5 cm2 is moderate stenosis, and a valve area 1.5–2 cm2 is mild stenosis. Mitral valve resistance is a useful research tool, although its clinical use lags behind mitral valve area. It is a measurement of the opposition to blood flow of the stenotic mitral valve and is computed by dividing pressure gradient by transvalvular flow. Mitral valve resistance has been advocated as being more accurate in patients with low cardiac output and low pressure gradients. The degree of MS can also be defined by the gradient across the mitral valve. By convention, a mean gradient >10 mm Hg at rest is severe MS, a mean gradient of 5–10 mm Hg at rest is moderate stenosis, and a mean gradient <5 mm Hg at rest is mild MS. Transvalvular gradient will increase in direct proportion to flow across the valve and thus conditions that increase cardiac output (e.g., anemia, hyperthyroidism, anxiety, exercise, MR) will result in increased gradients. Some patients may report significant exertional symptoms but have only a mild to moderate resting mitral valve gradient. In these patients, measuring hemodynamic response to exercise can be very helpful. With exercise, blood flow across the mitral valve increases and this can dramatically change the gradient. If the mitral valve pressure gradient increases with exercise and symptoms develop, the patient’s symptoms can be attributed to mitral valve disease (Figure 10.6). Figure 10.6 Exercise hemodynamics in a patient with MS. Transvalvular pressure gradient (simultaneous recording of LV and PCWP pressure tracings) at rest (a) and with exercise (b). The Gorlin formula is the standard for mitral valve area calculation (see text box). It was derived by the Gorlins (father and son) based on Torricelli’s law for flow across a round orifice [4]. An empiric constant was added (initially 0.7 and later changed to 0.85) to account for the difference between calculated and actual mitral valve areas.
Mitral stenosis
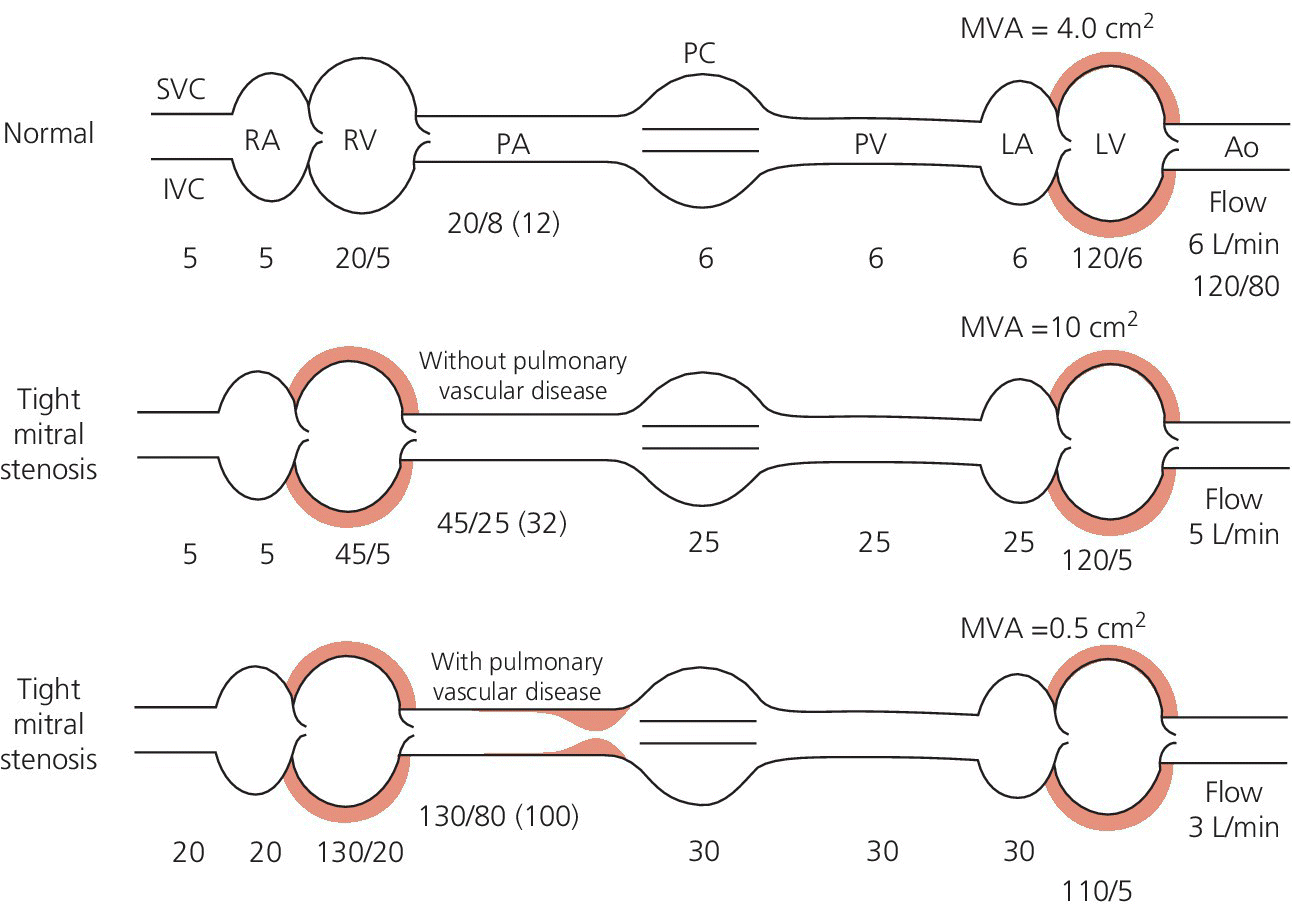
Cardiac hemodynamics in patients with MS
• Increased LA pressure
• Increased right heart pressures with exercise and/or at rest
• Prominent A wave on RA tracing
• Decreased slope of Y descent on RA tracing
• Gradient between LA and LV persists throughout diastole
Left atrium
Pulmonary artery
Left ventricle
Right ventricle
Cardiac output
Quantification of severity of MS
Calculating mitral valve area
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


