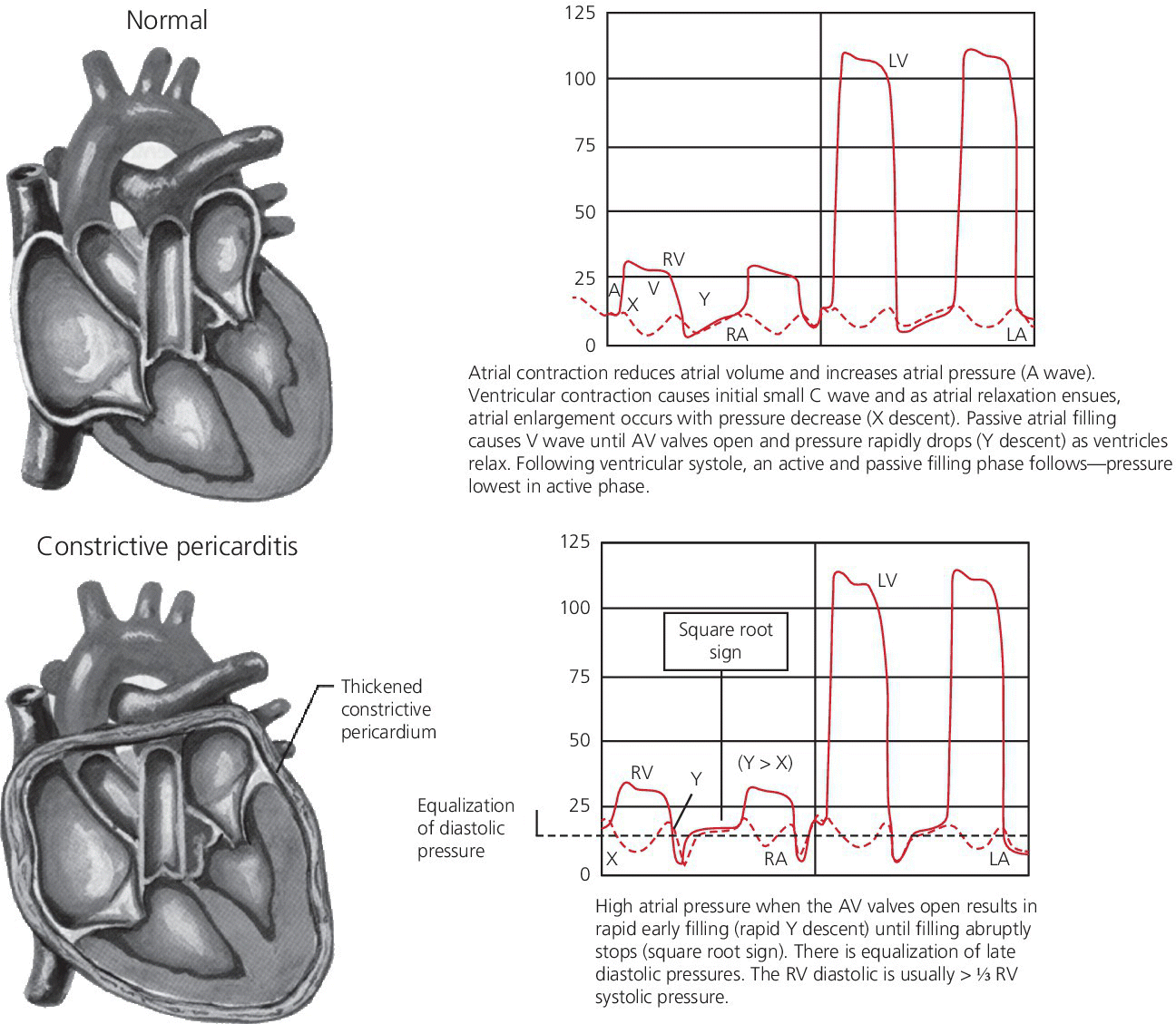
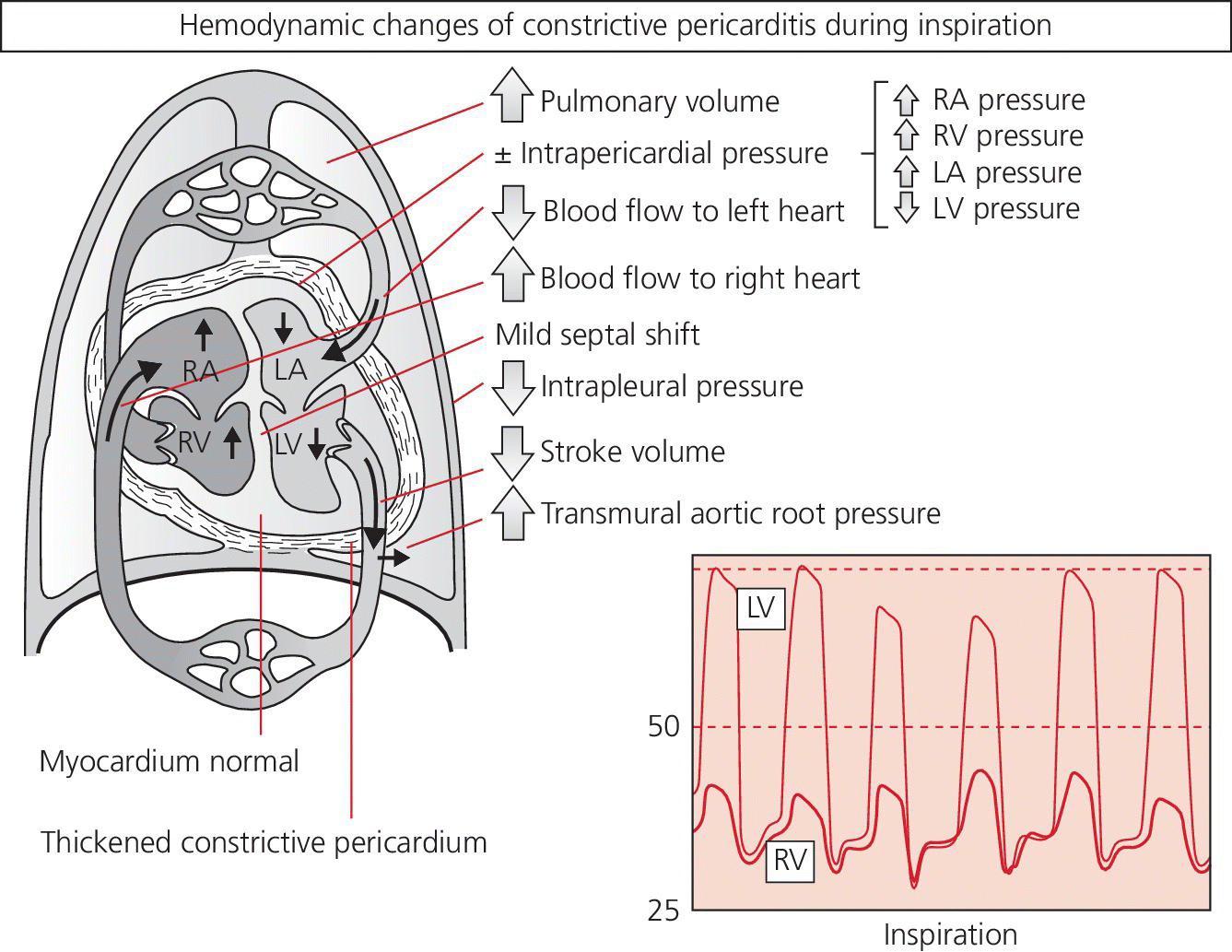
CHAPTER 18 David P. McLaughlin and George A. Stouffer The pericardium is a two‐layered sac that encircles the heart. The visceral pericardium is a mesothelial monolayer that is adherent to the epicardium, which is reflected back on itself at the level of the great vessels. The cardiac chambers are covered by the pericardium, with the exception of the left atrium and pulmonary veins, which are mostly outside of the pericardium. The parietal layer is a tough, fibrous outer layer. In the potential space that exists between these two layers there is normally 5–50 mL of serous fluid. The normal pericardium serves three primary functions: fixing the heart within the mediastinum, limiting the spread of adjacent infections, and limiting acute cardiac distention during sudden increases in intracardiac volumes. Constrictive pericarditis is a condition characterized by a dense, fibrous thickening of the pericardium that adheres to and encases the myocardium, resulting in impaired diastolic ventricular filling. The general paradigm is that constrictive pericarditis occurs over a period of years, due either to an acute insult (e.g., an infection) that elicits a chronic fibrosing reaction or to a chronic insult that stimulates a persistent reaction (e.g., renal failure). In the past the most common etiology was tuberculosis, but currently idiopathic pericardial constriction is the most frequent offender. Clinically, constrictive pericarditis is generally a chronic disease with symptom progression over a period of years. The clinical presentation is that of right‐sided heart failure and may resemble restrictive cardiomyopathy, cirrhosis, or cor pulmonale, among other conditions. Occasionally patients will go for years without the correct diagnosis being made. Recently, the advent of newer diagnostic technologies and a change in the predominant etiologies of constriction have led to increasing recognition of subacute presentations occurring over a period of months [1]. Pericardial constriction occurring after acute pericarditis of any cause is rare, but can occur early or later after the insult. Constrictive physiology can occur in the weeks following cardiac surgery or acute pericarditis. This often resolves and the possible transient nature must be carefully considered when planning treatment. In patients with permanent constrictive pericarditis, the presentation is usually years after the initial insult. Constriction following radiation therapy for malignancy can be a difficult diagnostic dilemma, as it can coexist with radiation‐induced restrictive myocardial disease. A study of 500 patients with acute pericarditis found that the risk of developing chronic constrictive pericarditis was low, but varied depending on the etiology. The incidence rate of constrictive pericarditis per 1000 person‐years was 0.8 cases for idiopathic/viral pericarditis, 4.4 cases for connective tissue disease/pericardial injury syndrome, 6.3 cases for neoplastic pericarditis, 31.7 cases for tuberculous pericarditis, and 52.7 cases for bacterial pericarditis [2]. Elevation of and equalization of diastolic pressures are the hallmarks of constriction (Table 18.1). Right atrial (RA), left atrial (LA), right ventricular (RV), and left ventricular (LV) diastolic pressures are elevated and nearly identical. In contrast to the normal heart, where pressures in the cardiac chambers are independent (i.e., unrelated) during diastole, in constrictive pericarditis the stiff pericardium limits expansion of the cardiac chambers. The chambers can fill beyond a certain limited point only by compressing other chambers and thus the diastolic pressures equalize. The ventricular waveform has a characteristic square root sign due to the rapid rise in early diastolic pressure prior to reaching the constraining effects of the rigid pericardium (Figure 18.1). Table 18.1 Hemodynamic findings in constrictive pericarditis. Figure 18.1 Ventricular pressure tracings in constrictive pericarditis. Simultaneous LV and RV tracings in a patient with constrictive pericarditis. Note the “dip and plateau” configuration and the near equalization during mid and late diastole. Operative findings in this patient included a dense, adherent pericardium. Central venous pressure dropped by approximately 15 mm Hg in the operating room with removal of the pericardium from the anterior RV and the RA. The differentiation of constrictive pericarditis from restrictive cardiomyopathy based solely on hemodynamics is difficult and will be discussed in more detail later in this chapter. Pseudoconstriction occurs when LV or RV volume overload happens rapidly, such that the pericardium does not have time to expand to accommodate the increased size. It is defined as constrictive‐appearing physiology in the absence of pericardial pathology, and as such is a diagnosis of exclusion. The following list gives the most common causes of pseudoconstrictive physiology. Bradycardia is listed but does not cause compressive physiology per se. It is mentioned here only in that the ventricular waveform can manifest a square root appearance in patients with heart rates in the 40s. Figure 18.2 Comparison of intracardiac pressures in the normal heart and in constrictive pericarditis. Figure 18.3 RA tracings from two patients with constrictive pericarditis. Note the elevated RA pressure, prominent Y descent, and lack of respiratory variation (both patients were breathing normally during these recordings). Figure 18.4 Effect of respiration on blood flow in mild constrictive pericarditis. The most obvious findings are related to elevated right heart pressures and include elevated CVP, hepatomegaly, ascites, and peripheral edema. The general physical examination in patients with constrictive pericarditis is not unlike that for those with right heart failure of any cause. There are, however, a few fairly specific physical findings that are important to recognize. Careful examination of CVP will invariably reveal elevation. Normally, inspiration leads to a fall in CVP, as the negative thoracic pressure generated in the thorax is transmitted to the right‐sided cardiac chambers. Kussmaul described a paradoxical rise in CVP with inspiration in patients with pericardial constriction that is rare in restrictive myocardial disease. Occasionally, prominent X and Y descents can be seen on examination of the neck veins. Friedreich’s sign, the sudden collapse of previously distended neck veins during early diastole, is found in patients with constrictive pericarditis. A high‐pitched early diastolic sound or pericardial knock occurring before a typical S3 is often heard. Plain chest X‐ray has some value in chronic pericardial constriction, as calcification of the pericardium can be seen in up to 25% of cases. Pericardial thickening can be seen on echocardiography, but must be distinguished from gain artifact or harmonics. Pericardial effusion is occasionally present and typically small. CT scanning and cardiac MRI are now the gold standard of cardiac imaging modalities used in diagnosing constriction. A pericardial thickness of >3 mm is suggestive of pericardial constriction, but it is important to note that pericardial thickening on imaging is absent in up to 20% of cases. Similarly, not all patients with thickened pericardium will have constrictive pericarditis, although a thickness of >6 mm adds considerable specificity to the diagnosis. Figure 18.5 Examples of concordance and discordance. Source: Courtesy of Cardiovillage.com. A study from the Mayo Clinic of 15 patients with constrictive pericarditis and 7 patients with restrictive cardiomyopathy provides some useful information [4]. Note, however, that this study was performed with high‐fidelity manometric catheters. The small, soft fluid‐filled catheters that are commonly used in cardiac catheterization laboratories can distort waveforms to a significant extent. Another study found that Kussmaul’s sign was neither sensitive nor specific for constrictive pericarditis. In a study of 135 patients, Kussmaul’s sign was present in only 21% of patients with surgically proven constrictive pericarditis [1]. Kussmaul’s sign may also occur in right‐sided heart failure, right ventricular infarction, and tricuspid stenosis. The two‐dimensional and Doppler echocardiographic features of constriction include a thickened pericardium, abnormal ventricular septal motion, flattening of the left ventricular posterior wall during diastole, respiratory variation in ventricular size, dilated inferior vena cava (IVC), impaired diastolic filling, and dissociation of intracardiac and intrathoracic pressures [5,6]. In constrictive pericarditis, there is a decrease in the mitral inflow velocity of greater than 25% with respiration. Also with respiration, variation in ventricular filling can sometimes be seen. During inspiration, a decrease in LV filling makes more room for RV filling, as the interventricular septum moves to the left and hepatic diastolic flow velocities increase during inspiration. During expiration, left ventricular filling increases, which decreases right heart filling and therefore hepatic diastolic forward flow velocity is decreased. In constriction, diastolic forward flow is usually greater than systolic forward flow. Additionally, hepatic diastolic flow reversal is increased, as the inflow across the tricuspid valve is interrupted by the pericardium and movement of the septum toward the right ventricle with expiration. Plethora of the IVC is common and failure of the IVC to decrease in diameter by 50% during the respiratory cycle is the echo equivalent of Kussmaul’s sign. M‐mode imaging can demonstrate an early diastolic notch of the interventricular septum corresponding to rapid cessation of filling, the pericardial knock, and the onset of the plateau phase of the ventricular diastolic waveform. Constrictive pericarditis and restrictive cardiomyopathy are very different diseases sharing a similar hemodynamic profile. Both have as the primary hemodynamic abnormality altered mid to late diastolic filling of the ventricles, leading to a syndrome of congestive heart failure. Often the heart failure is insidious in onset and predominantly right sided. These syndromes may mimic many other disease entities and it is common for both conditions to go undiagnosed for years. In the case of constrictive pericarditis, the impediment to filling is caused by the thickened unyielding pericardium. In restrictive cardiomyopathy, the abnormality is a result of a poorly compliant myocardium that limits the ability of the ventricles to expand and accept the filling volume of the atria. Rarely there is overlap and both entities can coexist (e.g., radiation‐induced myopericardial disease). The possibility of constriction or restriction should be entertained in any patient presenting with heart failure and normal systolic function (although systolic function will decline as restrictive cardiomyopathy progresses), particularly when other causes of this entity are not present. Although the differentiation of these two entities can be quite challenging to the clinician, a thorough understanding of both the similarities and differences is imperative to arriving at the correct diagnosis. Hemodynamic factors that are helpful in differentiating constrictive pericarditis from restrictive cardiomyopathy are listed in Table 18.2, and some generalities regarding the effects of respiration on hemodynamic findings in restrictive cardiomyopathy, constrictive pericarditis, and cardiac tamponade are shown in Table 18.3. Experience teaches that it is rare to arrive at a firm diagnosis of either restrictive cardiomyopathy or constrictive pericarditis based on hemodynamics alone. Table 18.2 Hemodynamic findings in constrictive pericarditis and restrictive cardiomyopathy. Table 18.3 Effects of respiration on hemodynamic findings in restrictive cardiomyopathy, constrictive pericarditis, and cardiac tamponade.
Constrictive pericarditis
Hemodynamics of constrictive pericarditis
Hemodynamic principles
Physical exam
Pericardial imaging techniques
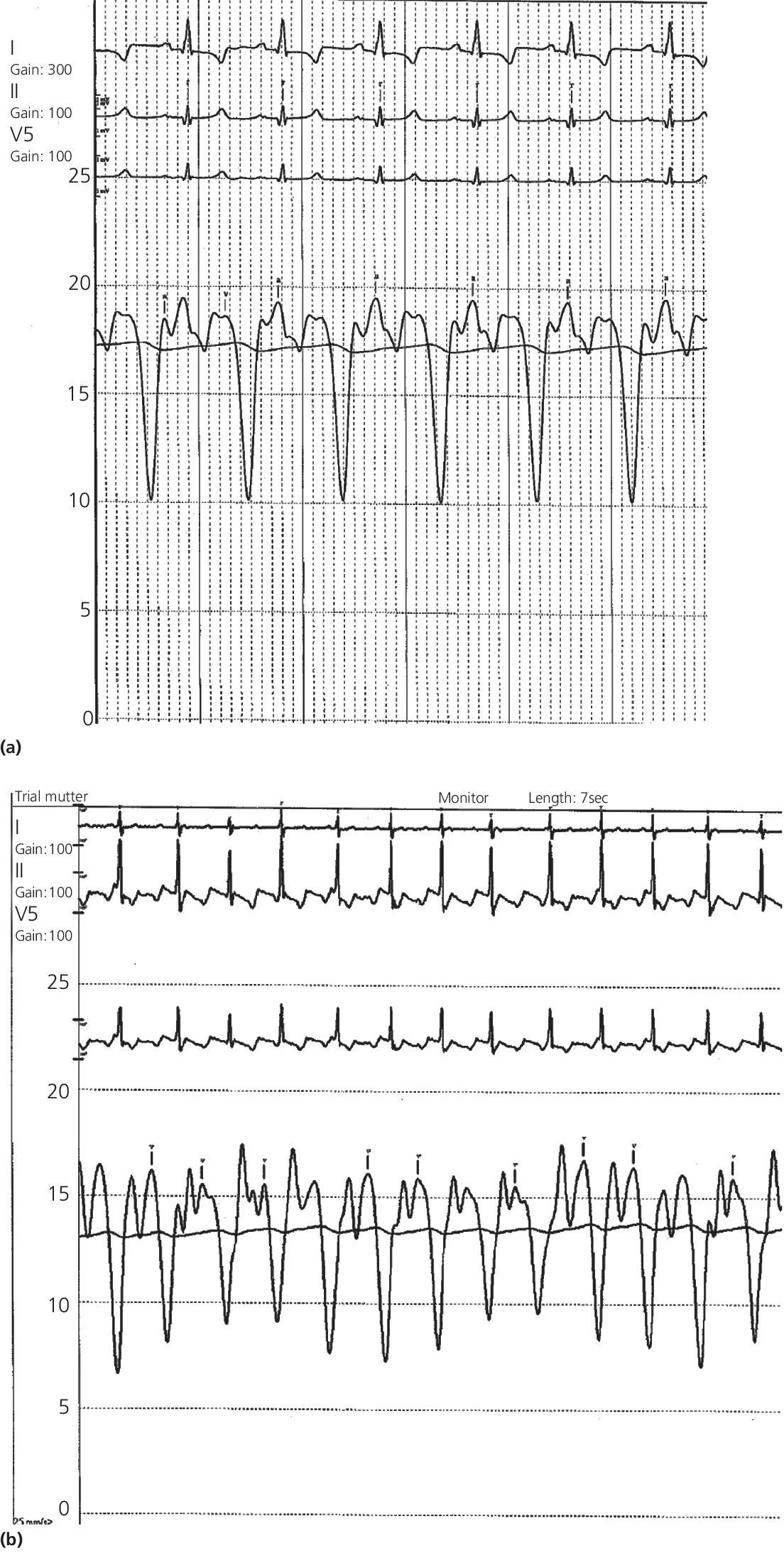
Findings at cardiac catheterization
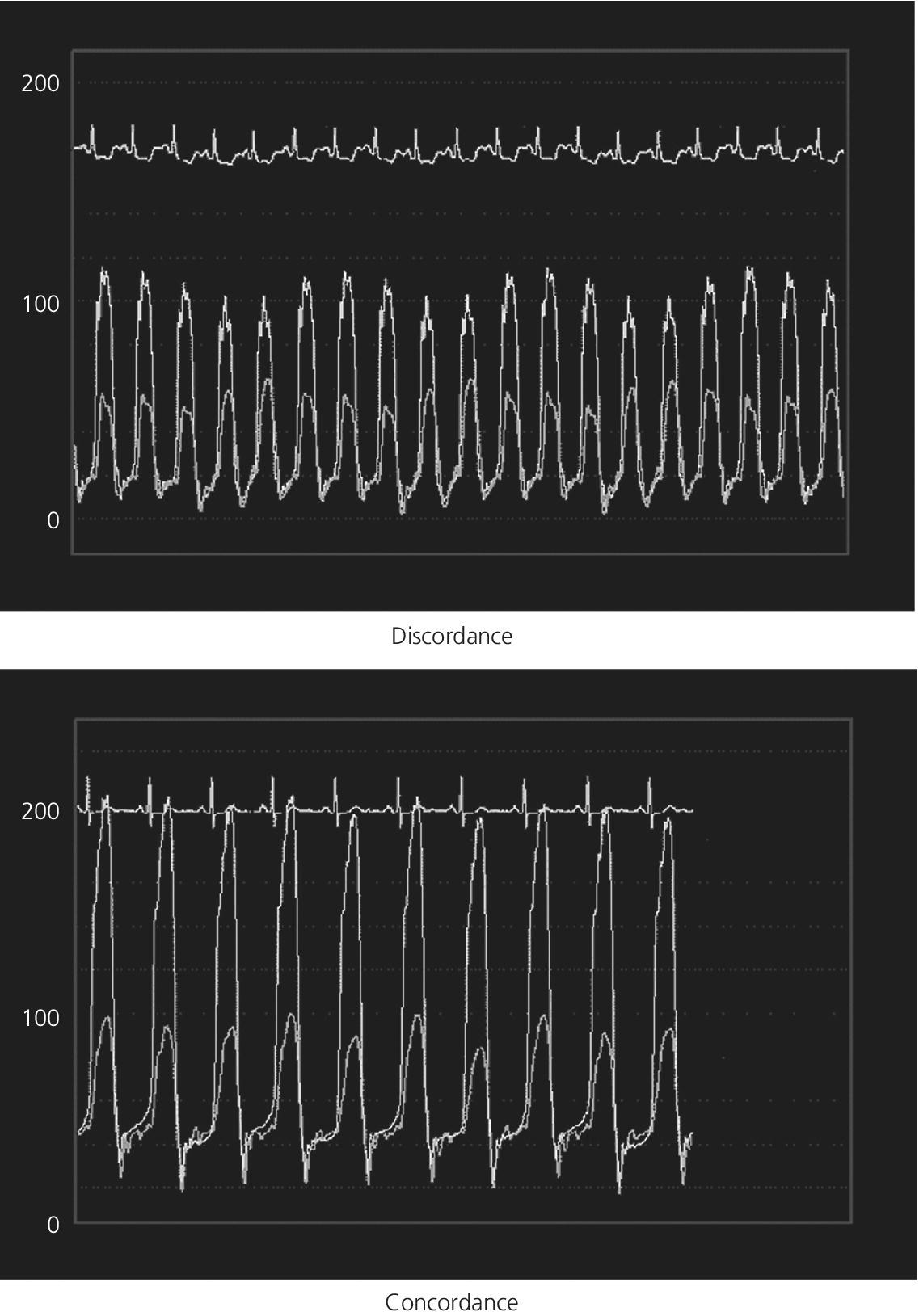
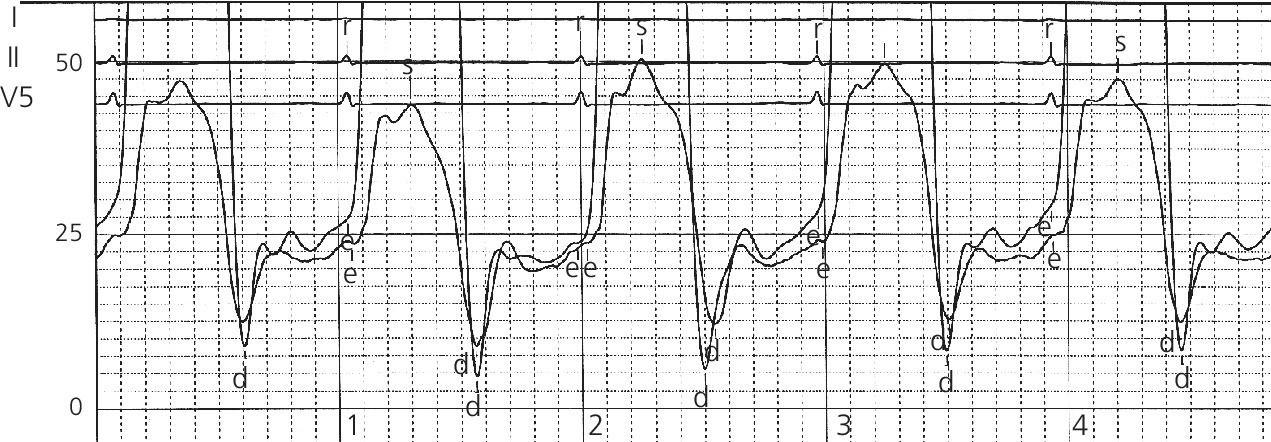
Sensitivity and specificity of various hemodynamic findings in constrictive pericarditis
Findings on echocardiography
Differentiation of constrictive pericarditis and restrictive cardiomyopathy
Constrictive pericarditis
Restrictive cardiomyopathy
LV systolic function
Usually normal
May be reduced
PA systolic pressure
Usually <50 mm Hg
May be >50 mm Hg
RV/LV systolic pressure
Discordant
Concordant
RVEDP/LVEDP separation
<5 mm Hg
>5 mm Hg
RVEDP/RV systolic pressure
>1/3
<1/3
Kussmaul’s sign
Present
Absent
Restrictive cardiomyopathy
Constrictive pericarditis
Cardiac tamponade
Intrathoracic pressure
Transmitted to intracardiac chambers and pulmonary venous circulation
Not transmitted to intracardiac chambers but to pulmonary venous circulation
Right‐sided chambers have increased filling during inspiration whereas left‐sided chambers have increased filling during expiration
The inferior vena cava shows little or no change on respiration
RA pressure
Decreases with inspiration
Increases or does not change with inspiration (Kussmaul’s sign)
Decreases with inspiration
Mitral inflow on echocardiography
No significant change in mitral E wave, deceleration time, or isovolumic relaxation time (IVRT) with phases of respiration
Marked respiratory variation in biventricular inflow
Decrease of >25% in mitral E wave velocity and prolonged IVRT on inspiration
Mitral valve E wave velocity decreases and IVRT increases by more than 30% on inspiration compared with expiration
Tricuspid valve E wave velocity increases with respiration
LV systolic pressure
Decreases with inspiration
Decreases with inspiration
Exaggerated decrease in LV systolic pressure with inspiration (pulsus paradoxus)
RV systolic pressure
Decreases with inspiration
Increases with inspiration
Increases with inspiration
Ventricular interdependence
Concordant
Discordant
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


