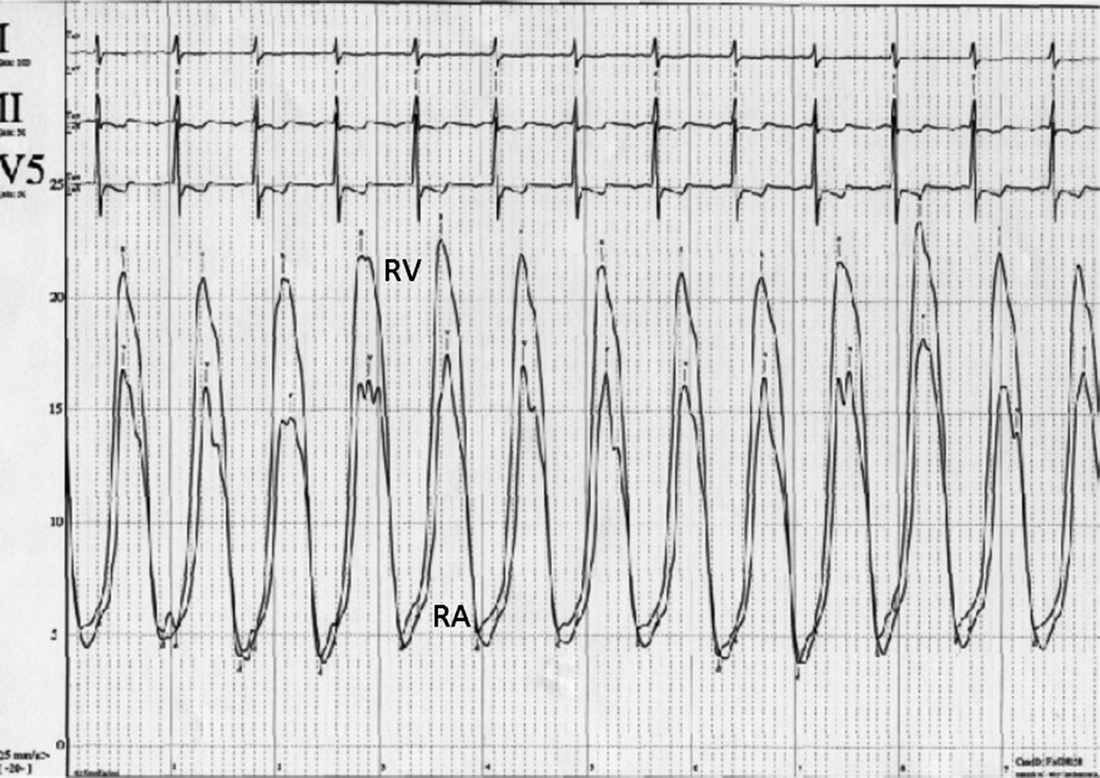
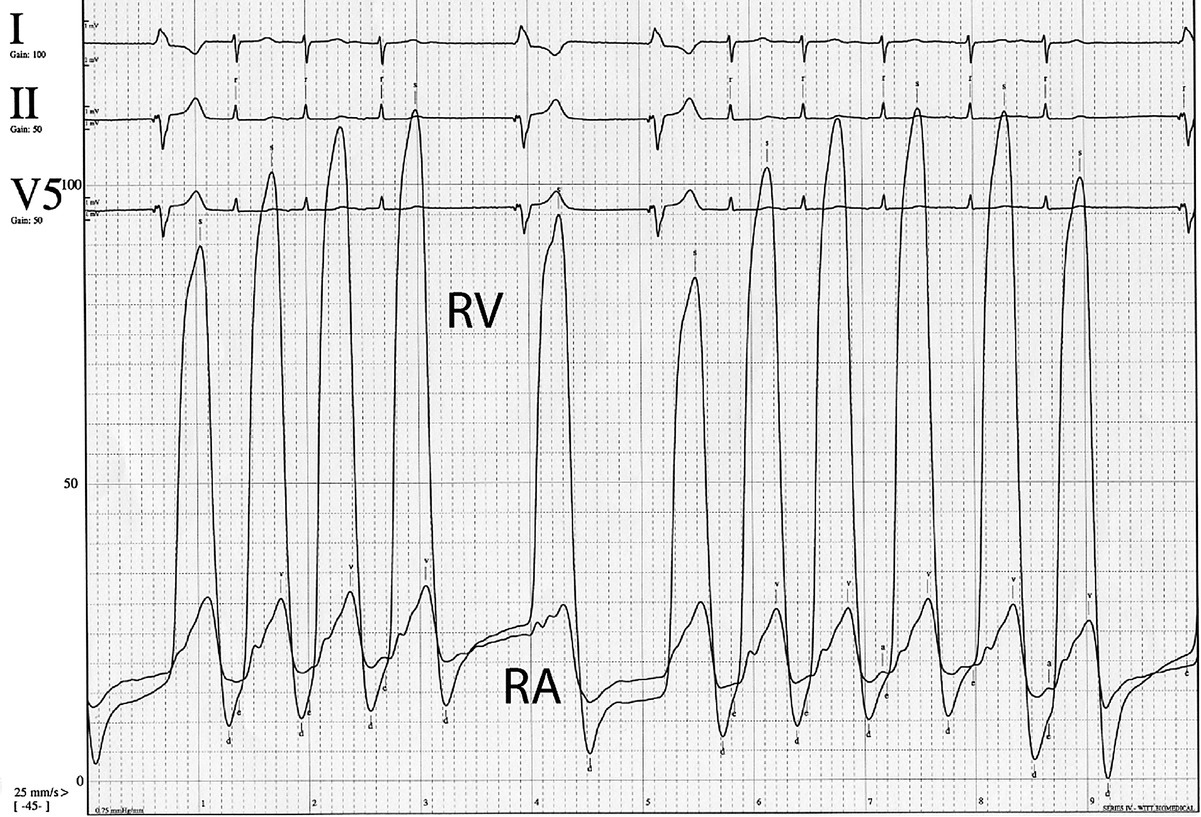
CHAPTER 13 David A. Tate and George A. Stouffer The tricuspid valve separates the right atrium (RA) from the right ventricle (RV). Its three leaflets are unequal in size, with the anterior leaflet usually being the largest. The leaflets are thin and translucent, a design that is adequate to the relatively lower pressures and hemodynamic stresses to which the right‐sided valves are subjected under normal circumstances (Figure 13.1). Indeed, although structural tricuspid valvular abnormalities do occur, hemodynamic perturbations involving the tricuspid valve are far more commonly seen in patients with morphologically normal valves subjected to abnormal hemodynamic stresses. Figure 13.1 Drawing showing tricuspid valve anatomy and pathology. Tricuspid regurgitation due to primary structural valvular abnormalities is rare, but will occasionally be seen due to rheumatic heart disease, myxomatous disease (prolapse), infective or marantic endocarditis, carcinoid heart disease, anorectic drugs, trauma, Marfan’s syndrome, or Ebstein’s anomaly. In addition, the tricuspid valve can be damaged during placement of leads for pacemakers or implantable cardioverter‐defibrillators or during right ventricular biopsies in heart transplant recipients. In contrast, secondary functional tricuspid regurgitation is commonly encountered as a consequence of conditions associated with increased pulmonary arterial pressures, such as left ventricular systolic or diastolic dysfunction, mitral regurgitation, mitral stenosis, primary pulmonary disease, or primary pulmonary hypertension. Dilatation of the tricuspid annulus may also be seen in right ventricular infarction or in dilated cardiomyopathies. The backward flow of blood from the RV to the RA is often clinically subtle due to the relatively compliant RA and the more conspicuous manifestations produced by the underlying primary disease process. Nevertheless, as the regurgitation becomes more severe, the signs and symptoms of elevated systemic venous pressure become evident and, in severe disease, cardiac output is diminished. Symptoms and signs are often due to associated left‐sided heart disease or pulmonary disease but, if there is significant tricuspid regurgitation, signs of this are generally evident on the physical exam. As with any cause of right heart failure, there are likely to be congestive findings on physical exam such as pedal edema, ascites, and hepatic enlargement. Inspection of the jugular veins will generally demonstrate both distention correlating with elevated RA pressure, and a distinct and prominent CV wave reflecting systolic regurgitant flow into the RA. The typical murmur is holosystolic and located at the left sternal edge. Augmentation of the murmur with inspiration helps to distinguish tricuspid from mitral regurgitation. Two‐dimensional (2D) and Doppler echocardiography are invaluable in the evaluation of tricuspid regurgitation [1]. The 2D portion of the study evaluates the structure of the valvular apparatus, but, as already noted, this is normal in the overwhelming majority of cases. RA and RV size, however, give important clues to the duration of the volume and pressure overload. The Doppler component of the study yields specific hemodynamic information. Color flow and pulse‐wave examination reveal the presence, direction, and magnitude of the regurgitant jet. Detection of systolic flow reversal in the inferior vena cava and hepatic veins is generally indicative of severe tricuspid regurgitation. Finally, continuous‐wave Doppler and the modified Bernoulli equation can be used to estimate the RV and pulmonary artery systolic pressures. In tricuspid regurgitation, the gradient between the RV and the RA during systole equals four times the square of the velocity. This gradient is then added to the estimated right atrial pressure (the jugular venous pressure) to estimate RV systolic pressure. In the absence of pulmonic stenosis, this also equals pulmonary systolic pressure. It is important to recognize that this calculation estimates the severity of the pulmonary hypertension, not the volumetric severity of the tricuspid regurgitation itself. In the cardiac catheterization laboratory, the findings associated with tricuspid regurgitation are most evident in the RA pressure tracing (Figure 13.2). The normal RA waveform consists of an A wave associated with atrial contraction, a C wave associated with ventricular contraction, and a V wave associated with rising atrial pressure just prior to opening of the tricuspid valve. With volumetrically important tricuspid regurgitation, there is a large systolic wave in the right atrial tracing, reflecting retrograde ejection of blood and consequent transmission of pressure from the RV to the RA. This waveform is variously termed an S wave or a CV wave, as it occurs during systole and subsumes the normally independent C and V waves. The magnitude of the systolic wave is determined by both the severity of the regurgitation and the compliance of the RA [2]. Figure 13.2 Simultaneous RA and RV pressures from a 67‐year‐old male who had undergone heart transplantation 9 years previously. With severe tricuspid regurgitation, the systolic wave in the RA becomes so prominent that the tracing resembles the right ventricular tracing (Figures 13.2 and 13.3). Indeed, in very severe tricuspid regurgitation the RA and RV pressure tracings are virtually identical, reflecting the fact that in the absence of a competent tricuspid valve, the RA and RV become, functionally, a single chamber. While this may be most evident with the simultaneous placement of two fluid‐filled catheters in the RA and RV (Figures 13.2 and 13.3), this is procedurally cumbersome and the catheter across the tricuspid valve introduces the possibility of artifactual tricuspid regurgitation. Careful performance and observation of RV to RA “pullback” pressures, paired with Doppler and echocardiographic analysis, can provide compelling evidence for severe tricuspid regurgitation. Similarly, while right ventricular angiography can help assess the severity of tricuspid regurgitation, it is rarely necessary. Moreover, the catheter across the tricuspid valve and the ectopy associated with right ventricular contrast injection often introduce artifactual error. With current echocardiographic and Doppler techniques, angiography generally is of little additional utility. Source: Rao 2013 [2]. Reproduced with permission of John Wiley and Sons. Figure 13.3 Simultaneous RA and RV pressure in a patient with severe tricuspid regurgitation. Catheterization data do not allow definitive distinction between structural and functional tricuspid regurgitation. However, severe tricuspid regurgitation associated with relatively low RV and PA systolic pressures (less than 40 mm Hg) are more likely to be at least partially due to organic valvular disease. On the other hand, tricuspid regurgitation associated with very high RV systolic pressures is much more likely to be functional. In the case of functional tricuspid regurgitation, the mainstay of therapy is treatment of the condition causing pulmonary hypertension. This may be medical therapy as, for example, in the setting of left ventricular failure, or procedural as, for example, in the setting of mitral stenosis. Diuretics may be useful for refractory fluid retention. With structural valve disease, tricuspid valve repair or replacement is appropriate for patients who are either refractory to medical therapy or undergoing surgery for coexistent mitral valve disease. Often a prosthetic ring is used for annuloplasty. If valve replacement is necessary, bioprostheses are favored because the tricuspid valve may be relatively prone to thrombosis. Acquired tricuspid stenosis is uncommon. Most cases are due to rheumatic heart disease, carcinoid heart disease, or stenosis of a prosthetic valve initially placed for tricuspid regurgitation. When rheumatic tricuspid stenosis is present, it is generally associated with mitral stenosis, which accounts for most of the presenting signs and symptoms. The signs and symptoms of tricuspid stenosis may be mimicked by tumors (myxoma or metastasis) or vegetations that obstruct RV inflow. The signs and symptoms of tricuspid stenosis are primarily due to increased systemic venous pressure. Peripheral edema, ascites, hepatic enlargement, and right upper quadrant discomfort may develop with chronic tricuspid stenosis or regurgitation. Decreased cardiac output may cause pronounced fatigue. Jugular venous pressure is increased and, if the patient is in sinus rhythm, there is a prominent A wave due to impaired RV filling during atrial systole. However, due to the increased right atrial pressure, these patients are often in atrial fibrillation. Though clinically subtle, the finding of a blunted or absent Y descent supports the diagnosis. The murmur of tricuspid stenosis is a low‐pitched diastolic murmur at the lower left sternal edge, although this is often obscured by or difficult to differentiate from the usually associated mitral stenosis murmur. Accentuation of the murmur during inspiration may help to identify a component of tricuspid stenosis, even if there is concurrent mitral stenosis. Echocardiography typically reveals thickened tricuspid leaflets, decreased mobility, scarred chordae, and sometimes doming of the leaflets if they remain pliable. Carcinoid heart disease is associated with a distinctive morphology of a thickened tricuspid valve that is narrowed and fixed in the open position. The pandiastolic gradient of tricuspid stenosis is reflected in both the slow E to A slope of the M‐mode echocardiogram and in the slowly falling velocity of the turbulent flow on Doppler assessment. Doppler evaluation also allows estimation of the diastolic pressure gradient by the modified Bernoulli equation. With modern echocardiographic and Doppler techniques, cardiac catheterization is generally not necessary for the diagnosis of tricuspid stenosis. When the diagnosis is in doubt, however, careful invasive determination of the transvalvular gradient is necessary. In most cases, a careful RV to RA pullback will reveal the gradient. However, the gradients are generally small, in the range of 4–8 mm Hg. If the cardiac output is low, tricuspid gradients are particularly likely to be low and may not be adequately evaluated with a catheter pullback. In this case, separate simultaneous catheters should be placed in the RA and RV. Clinically, significant tricuspid stenosis is usually associated with a valve area of 1.5 cm2 or less. Treatment of tricuspid stenosis includes diuretics and occasionally nitrates to relieve venous congestion. Refractory patients can undergo tricuspid valve replacement, but in most cases the concomitant mitral valve disease primarily determines the indication and timing of surgery. A surgical approach may also be indicated for debulking of obstructive tumors or myxoma. The early experience with percutaneous balloon valvuloplasty for tricuspid stenosis is encouraging.
The tricuspid valve
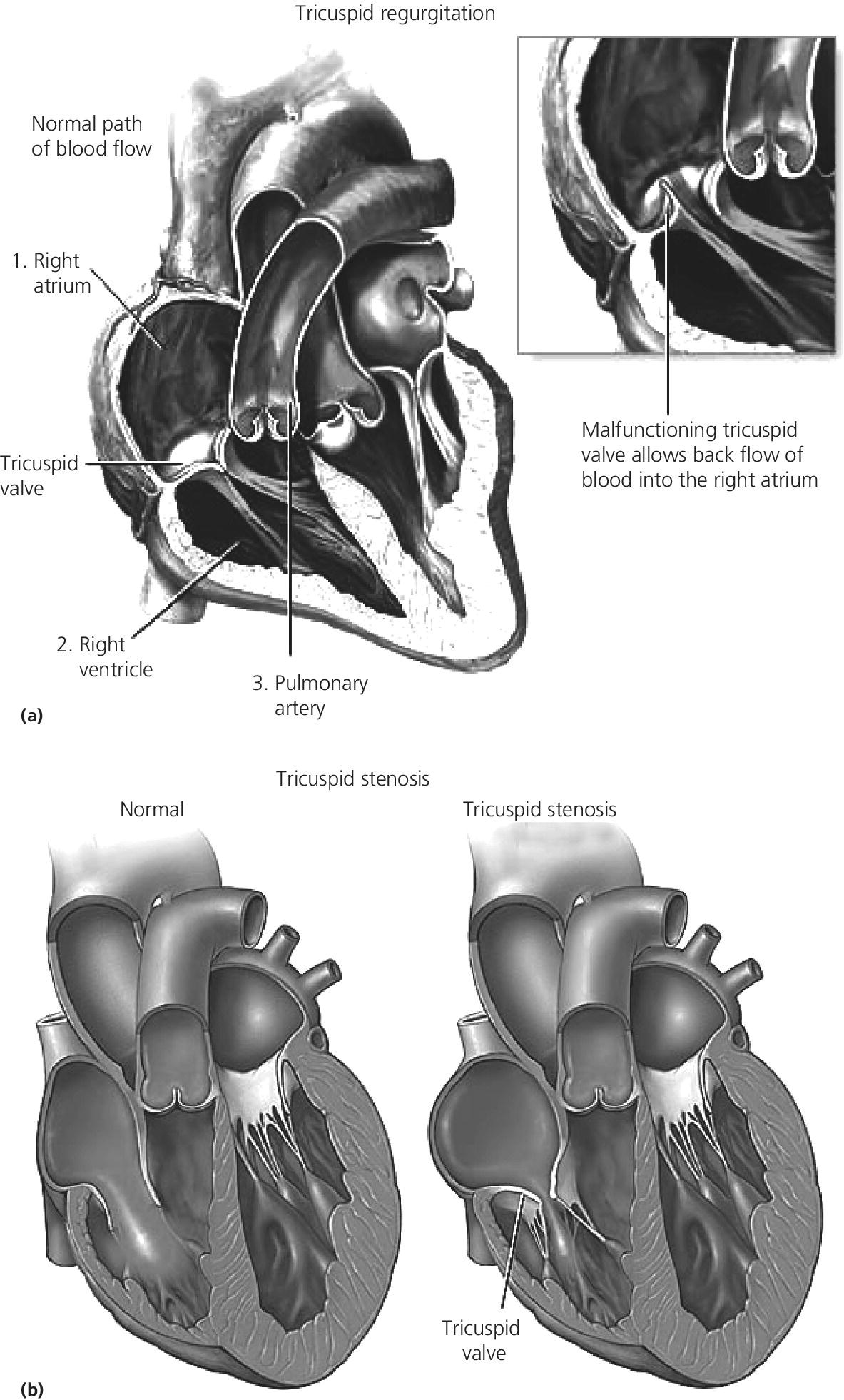
Tricuspid regurgitation
Pathophysiology
Hemodynamic changes detected by physical exam
Hemodynamic changes detected by echocardiography
Hemodynamic changes evident at catheterization
Treatment
Tricuspid stenosis
Pathophysiology
Hemodynamic changes detected by physical exam
Hemodynamic changes detected by echocardiography
Hemodynamic changes evident at catheterization
Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


