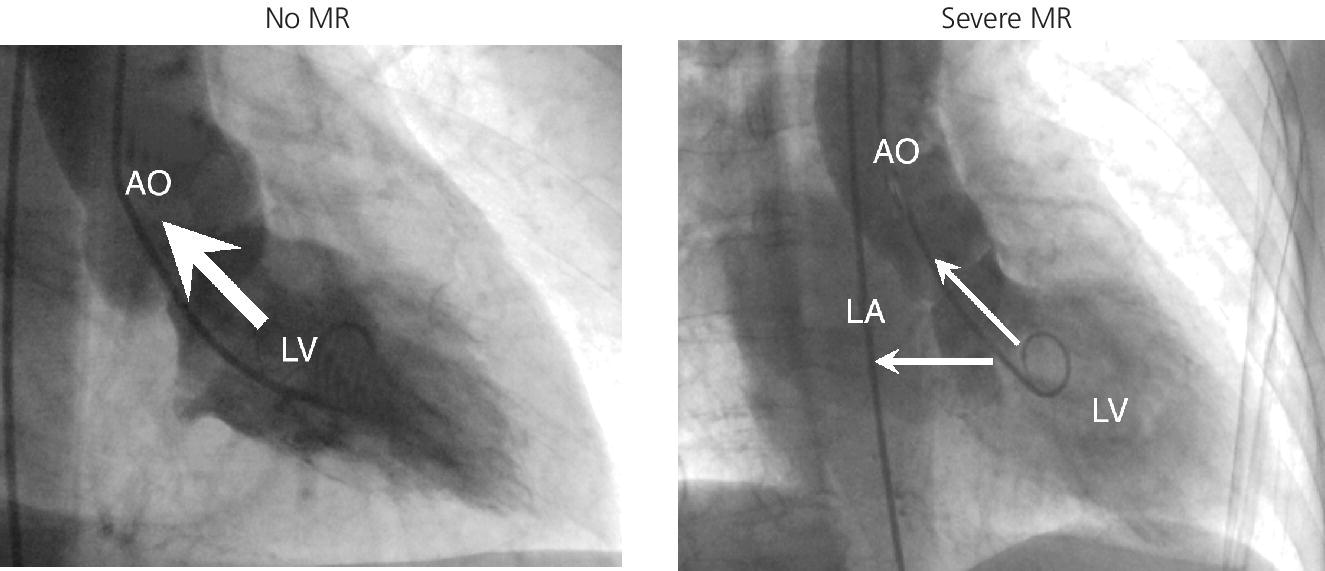
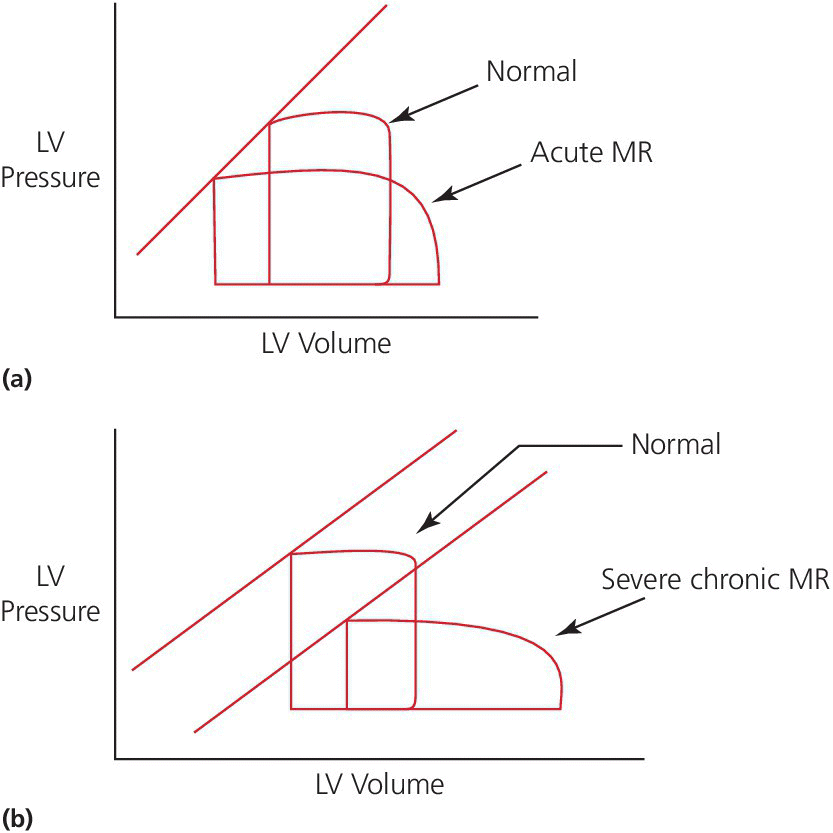
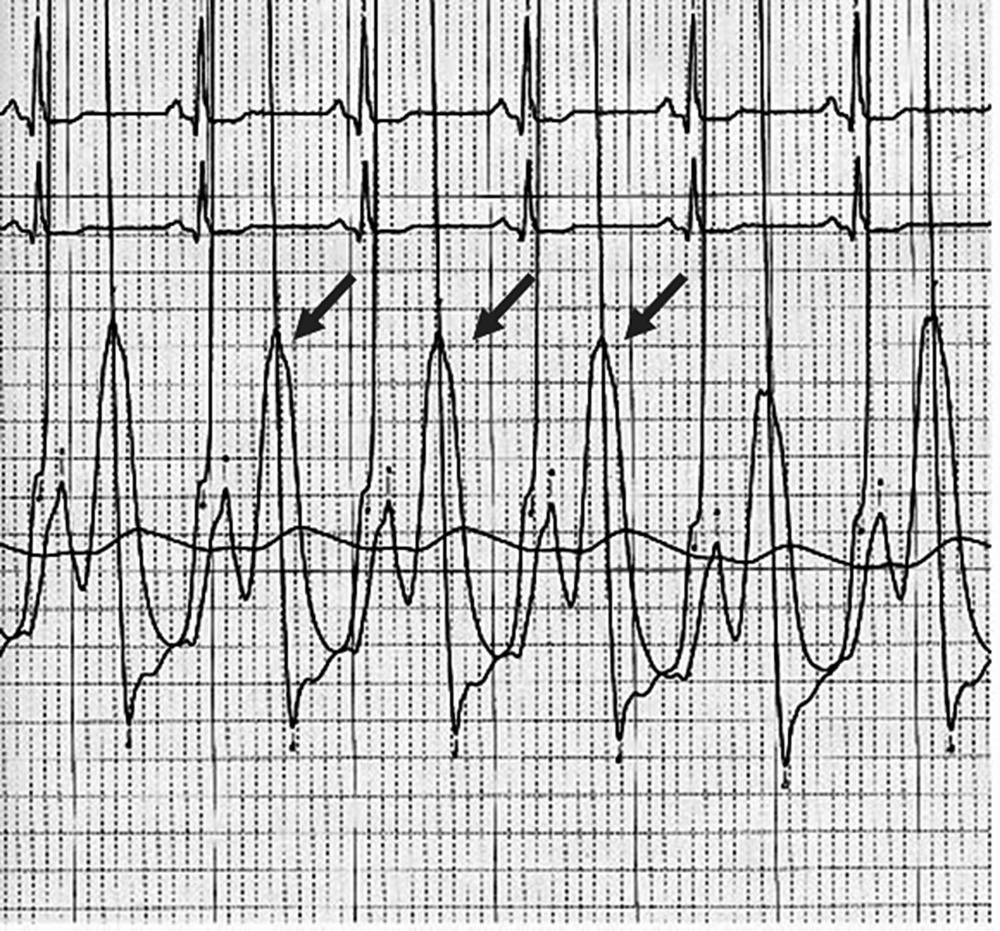
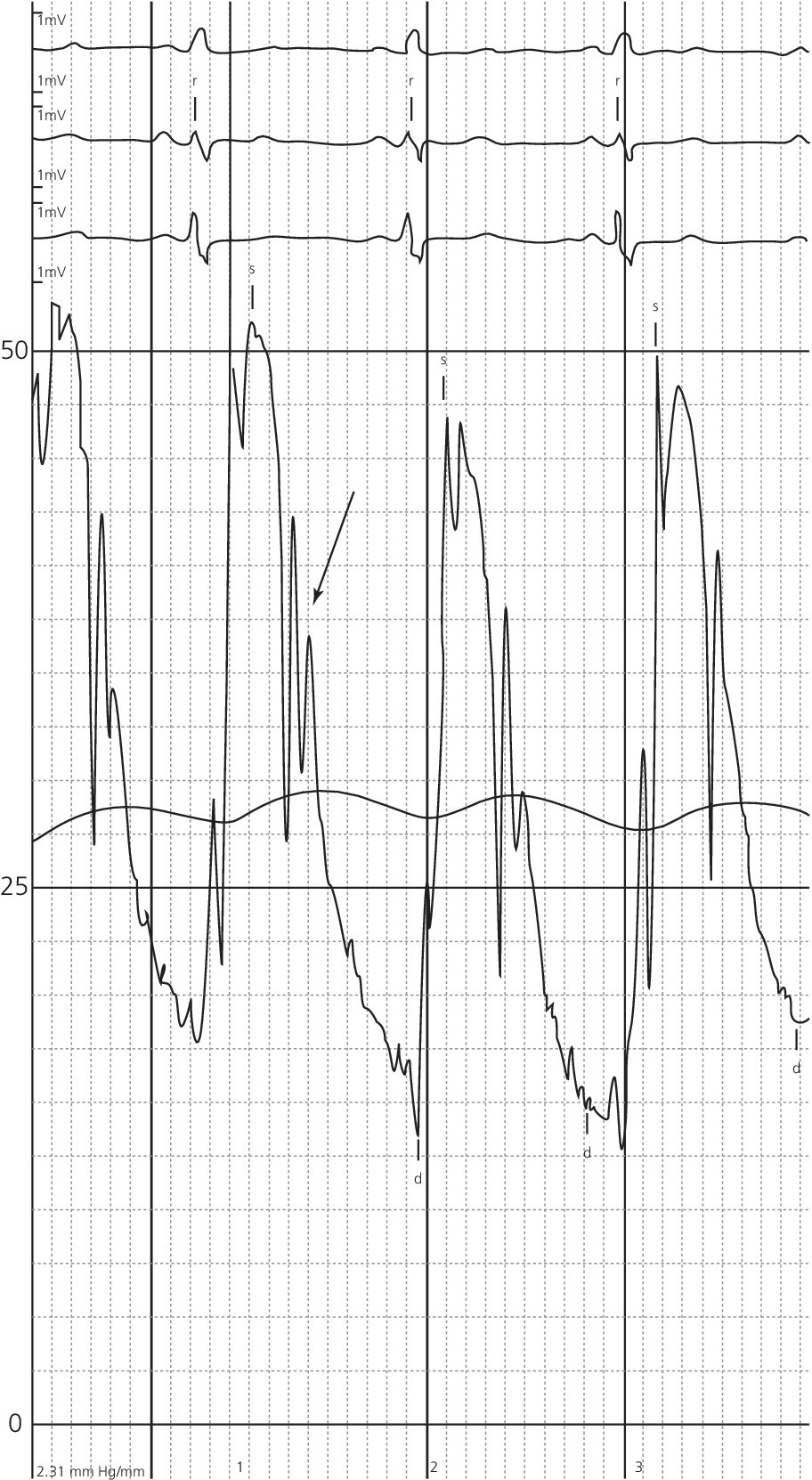
CHAPTER 12 Robert V. Kelly, Mauricio G. Cohen and George A. Stouffer Patients remain asymptomatic for years with chronic severe mitral regurgitation (MR) before developing exertional dyspnea. In contrast, sudden onset of pulmonary edema is a hallmark of acute MR. The natural history of chronic MR is variable and depends on the regurgitant volume, left ventricular (LV) function, and the underlying cause of MR. Compensatory mechanisms enable the patient to adapt to severe MR (Figure 12.1). The development of symptoms at rest in chronic MR can be an ominous finding, especially if coupled with decrease in LV systolic function. Figure 12.1 Mitral regurgitation. Still frames from left ventriculograms from two patients showing no mitral regurgitation on the left and severe mitral regurgitation on the right. In both cases, dye has been injected into the left ventricle. Note that the left atrium is opacified on the right but not on the left, indicative of mitral regurgitation. The arrow represents blood flow. With a competent mitral valve, all of the blood ejected by the left ventricle goes into the aorta. In patients with mitral regurgitation, a portion of left ventricular stroke volume goes into the left atrium. [Ao = aorta; LA = left atrium; LV = left ventricle.] MR can be caused by structural abnormalities of the mitral leaflets, papillary muscles, chordae tendineae, or mitral annulus. A partial list of the causes of MR includes myxomatous changes, congenital abnormalities, chordal rupture, papillary muscle dysfunction or rupture secondary to myocardial ischemia or infarction (MI), endocarditis, trauma, and rheumatic degeneration. In patients with MR due to myocardial ischemia or infarction, the posterior leaflet is most likely to be incompetent, as the posterior papillary muscle is supplied solely by the posterior descending artery, whereas the anterolateral papillary muscle has a dual blood supply, including diagonal branches of the left anterior descending coronary artery and often obtuse marginal branches from the left circumflex artery. MR can occur in the setting of a normal mitral valve and apparatus when there is pathology of the mitral annulus. In healthy adults, the annulus is about 10 cm in circumference. It is a soft and flexible structure, which contributes to valve closure by enhancing valvular constriction, due to the contraction of the surrounding LV muscle. Dilation of the left ventricle can cause dilatation of the mitral valve annulus and mal‐apposition of the mitral leaflets. Mitral annular calcification (MAC) can also cause regurgitation in severe cases. MAC is associated with hypertension, diabetes, aortic stenosis, Marfan’s syndrome, Hurler’s syndrome, and chronic renal failure. In severe calcification, a rigid ring of calcium encircles the mitral orifice and calcific spurs may project into the adjacent myocardium. Severe mitral annular calcification may also immobilize the mitral leaflets, resulting in MR. Acute MR is a rare but potentially life‐threatening condition that is generally associated with abrupt onset of dyspnea, heart failure, and shock. Characteristic hemodynamic changes include increased LV preload, increased total stroke volume, compromised forward stroke volume, decreased LV end‐systolic diameter, and increased ejection fraction. Causes of acute MR include ruptured papillary muscles (e.g., during acute MI) or rupture chordae (e.g., from myxomatous disease), myocardial ischemia leading to papillary muscle dysfunction, bacterial endocarditis, and trauma. Acute MR is characterized hemodynamically by regurgitation in the absence of any compensatory dilation of a relatively noncompliant left atrium or left ventricle. The lack of time for compensatory mechanisms to develop results in an abrupt rise in left atrial (LA) pressure, which is generally accompanied by large V waves on pulmonary capillary wedge pressure (PCWP) tracing, appearance of large V waves in the PA tracing (so‐called Camelback PA tracing, in which V waves are reflected through a compliant pulmonary vasculature; see Figure 12.4) and a rapid Y descent as the distended LA quickly empties. The hemodynamic changes of chronic MR can be predicted by understanding the compensatory changes that occur. The heart adapts to chronic MR primarily by LA and LV dilation. The LV adapts to substantial regurgitant volumes by increasing LVEDV to maintain adequate forward cardiac output. According to Laplace’s law (wall tension is related to radius × intraventricular pressure), the increased LVEDV increases wall tension to supranormal levels. In chronic MR, LVEDV and LV mass increase, usually in proportion to the degree of LV dilatation. The degree of hypertrophy correlates with the amount of chamber dilation so that the ratio of LV mass to end‐diastolic volume remains in the normal range (in contrast to the situation in patients with LV pressure overload). At the same time there is a greater volume at a given pressure, resulting in a shift in the pressure–volume relationship (Figure 12.2). In most patients, LV compensation is maintained for years, but eventually LV failure occurs. This results in an increase in preload and LV end‐systolic volume (LVESV), and a decrease in LVEF and stroke volume. Figure 12.2 Pressure–volume loops in acute MR (a) and chronic MR (b). LVESV is an important pre‐operative prognostic marker, especially in terms of mortality, post‐operative heart failure, and post‐operative LV systolic function. LVESV index is an important marker of LV function in MR patients and helps with mitral valve surgery decisions. End‐systolic diameter (ESD) on echo is also a useful prognostic indicator. An ESD >45 mm is generally used as an indication for surgery, although if mitral valve repair can be accomplished a lower cutoff value has been advocated. The transition from a compensated state to a decompensated state in chronic MR is not completely understood. LV systolic function begins to decline, LVEDV increases, and filling pressures rise. Thus, chronic decompensated MR results in both systolic and diastolic dysfunction. The primary findings at catheterization in chronic MR include: Figure 12.3 Simultaneous LV and PCWP tracing in a patient with severe MR showing a V wave (arrow). Figure 12.4 Pulmonary artery tracing in a patient with severe MR (the same patient as in Figure 12.3) showing bifid appearance characteristic of a reflected V wave (arrow). The pulse is sharp in severe chronic MR and the pulse volume is usually normal. The apical impulse is brisk and hyperdynamic. It is often displaced to the left and can be associated with a prominent LV filling thrill. On auscultation, the first heart sound is diminished in severe MR. A wide splitting of S2 is common. It results from the shortening of the LV ejection and an earlier aortic valve closure as a consequence of reduced resistance to LV outflow. If severe pulmonary hypertension develops, P2 can be louder than A2. There is a pansystolic murmur. In severe MR, it commences immediately after a soft S1. It may extend beyond A2 because of the persisting pressure difference between the LV and LA after aortic closure. The murmur is a blowing high‐pitched murmur and is loudest at the apex. It often radiates to the axilla, but it may also radiate to the sternum or aortic valve area if the posterior leaflet is involved, mimicking aortic stenosis. The murmur of MR is usually accentuated by isometric exercise. In papillary dysfunction, the MR murmur may occur later in systole, with preservation of a normal S1 because the initial closure of the mitral valve is unaffected. A third heart sound but not a fourth heart sound is typical of chronic MR. In MR caused by mitral valve prolapse and myxomatous degeneration of the valve, the murmur may not be holosystolic, but rather tend to be more early or mid‐systolic and have a more musical quality. In acute severe MR, the patient is almost always symptomatic and often in heart failure. A systolic murmur will be present, although it may not be holosystolic and may disappear in mid or late systole if left atrial pressure is elevated. The murmur of acute MR is generally lower in pitch and softer than the murmur of chronic MR. A S3 and S4 are common, but there may not be a hyperdynamic apical impulse if the ventricle is normal in size. The mitral valve, valve apparatus, chordae, and papillary muscles can be visualized on echocardiography. The LA and LV size can be measured. Severity of MR can be defined by several different echo parameters. The intensity of the Doppler signal, the ratio of the regurgitant flow (RF) to the forward flow (FF), the regurgitant jet area, the ratio of regurgitant jet to LA area, and effective regurgitant orifice (ERO) all correlate with the severity of MR. Reversal of Doppler flow in pulmonary veins during systole can also be assessed and is an important indicator of severe MR.
Mitral regurgitation
Pathology
Acute MR
Hemodynamic concepts in patients with chronic MR
Compensatory mechanisms in chronic MR
Cardiac catheterization and MR hemodynamics
Physical examination
Echocardiography
Important points
Hemodynamics of mitral regurgitation
Acute
Chronic compensated
Chronic decompensated
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


