Prosthetic Valves
Before the development of cardiopulmonary bypass in the late 1950s, surgical attempts to correct valvular pathology were limited to closed valvotomy first performed on the aortic valve by Theodore Tuffier in 1912 and on the mitral valve by Elliot Cutler in 1923. In 1954, Charles Hufnagel designed the first mechanical valve, which he implanted in the descending aorta in patients with aortic insufficiency.1 The turning point in valvular surgery was the availability of cardiopulmonary bypass, which enabled surgeons to correct valve lesions under direct vision.2 Dwight Harken performed the first successful aortic valve replacement in the subcoronary position in 1960,3 and the same year Nina Braunwald implanted a ball and cage prosthesis designed in her laboratory in the mitral position.4 Working with Lowell Edwards, a retired engineer, Albert Starr, led the way from these isolated reports to an era of routine valve surgery by developing a ball valve to industrial quality standards and by solving the problem of how to secure the prosthesis to the recipient tissues.
By 1968, more than 2000 Starr-Edwards ball and cage valves had been implanted,4 but the persistent risk of thrombotic and hemorrhagic complications was a major incentive underpinning efforts to establish a nonthrombogenic alternative. Homograft valves developed by Donald Ross5 after Carlos Duran’s work were limited by problems of availability so that attention was directed toward pulmonary autograft by Ross6 and the use of porcine7 valves first implanted by Binet et al. Early failures with the porcine valve stimulated the use of alternative tissues, such as pericardium, fascia lata, and dura mater, which were used as substrates to manufacture valve prostheses. Despite attempts to increase longevity by fixation in formaldehyde, these valves showed signs of structural degeneration within a few years, and it was not until Alain Carpentier’s breakthrough discovery in the mid 1960s of the ability of glutaraldehyde fixation to yield a much more durable valve, that tissue valves became a valuable alternative to mechanical valves.8 Carpentier used stent-mounted porcine valves to facilitate implantation and coined the term bioprosthesis to reflect the biological origin and the prosthetic nature of these valves.9 The term xenograft used in the past is scientifically inappropriate. A graft is made from a living or fresh or cryopreserved material. Its fate is based on the survival of the cells or on host cells ingrowth. A bioprosthesis is made from chemically treated tissues, the durability of which is based on the stability of the tissue, not on cell regeneration.12 The main advantage of a bioprosthesis over a mechanical valve is the lower risk of thromboembolism, avoiding the need for anticoagulation. Starr advised Edwards’ laboratory, the manufacturer of his own prosthesis, to develop a tissue valve with Carpentier; the Carpentier-Edwards porcine valve remains one of the most widely used prostheses today and is the model on which most bioprostheses are still based10 (incorporating features such as glutaraldehyde tissue fixation and mounting into a metallic stent to facilitate implantation).
Although both the Carpentier-Edwards bioprosthesis and the Starr-Edwards ball and cage valve had good outcomes, the search for a nonthrombogenic, permanent solution for valvular heart disease continued. The principles and techniques of mitral valve reconstruction developed by Carpentier through the 1970s to challenge his own valvular bioprosthesis established mitral valve reconstruction as the optimal alternative to valve replacement in mitral disease.11,12 Repair was found to be less satisfactory, however, in most aortic valve pathology because of the limited surface of coaptation between leaflets. Donald Ross developed a new operation in which he used the patient’s own pulmonary valve to replace the diseased aortic valve and replaced the pulmonary valve with an aortic homograft in the hope that the durability of the homograft in the pulmonary position would be better than in the aortic position.6 The Ross operation remains a valuable alternative in young patients, but the relative complexity of the procedure and inferior durability compared with mechanical valve replacement mean that this operation represents only a small proportion of valve replacement procedures performed worldwide.
In the ball and cage model, the ball material was changed from Silastic to Stellite in the 1970s, and cloth was added to the sewing ring. Tilting disc prostheses rapidly established themselves as the second generation of mechanical valves, followed in the mid 1970s by bileaflet prostheses, which, because of a superior hemodynamics and low profile, rapidly became the most widely used mechanical prosthesis.10 Parallel advances in tissue fixation and anticalcification treatments, described in more detail later, saw bioprostheses evolve from the first-generation bioprosthetic valves through more durable second-generation valves with antimineralization treatments to third-generation bioprostheses with improved glutaraldehyde fixation, which now represent the most commonly implanted valves worldwide. In parallel, stentless valvular bioprostheses were introduced in 1988 in a bid to secure better hemodynamic profile from bioprostheses.13
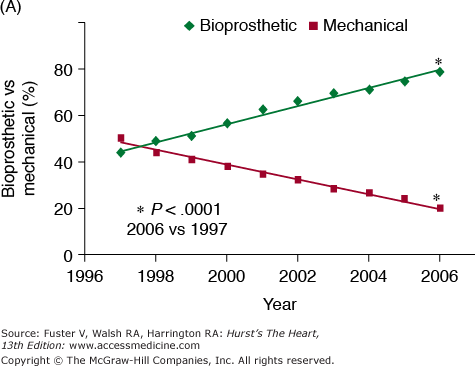
Although valve replacement may be performed using the patient’s own pulmonary valve (autograft) or cadaveric aortic valve (homograft), this chapter focuses on the description of and indications for prosthetic valves, which represent the vast majority of valve replacements carried out worldwide. Prosthetic valves are divided into mechanical and bioprosthetic valves (Table 80–1). The range of alternatives reflects the drawbacks of the available choices which, despite almost half a century of concentrated effort, remains the issue of valve deterioration, requiring reoperation associated with bioprosthetic valves, versus the thrombotic and bleeding complications associated with mechanical valves. Figure 80–1 is adapted from a registry analysis of aortic valve replacement in the United States and summarizes the relative numbers of bioprosthetic and mechanical models implanted in the United States between 1997 and 2006.14 This is described in more detail in the aforementioned comprehensive review of the relative performance of prosthetic valves by Grunkemeier et al,10 which lists the approximate numbers of each valve implanted since their introduction until 1998, based on estimates provided by the relevant valve manufacturers.
| Valve Type | Examples | Characteristics | Comments | |
|---|---|---|---|---|
| Mechanical | Ball and cage | Starr-Edwards | Titanium cage, Silastic ball | High profile Potential for LVOTO in the mitral position Moderately thrombogenic Good long-term durability data Not commonly used today |
| Tilting disc | Medtronic-Hall Bjork-Shiley | Titanium housing, carbon leaflet | High profile Potential for interference with opening if malpositioned in small aorta Thrombogenic | |
| Bileaflet | St. Jude | Pyrolite disc and housing | Lowest profile Less thrombogenic Best hemodynamic profile, particularly in small sizes Most commonly used mechanical prosthesis today | |
| Bioprosthesis | Porcine | Carpentier-Edwards Hancock Standard | Glutaraldehyde fixed pig aortic valve on titanium stent | Nonthrombogenic Risk of postoperative transvalvular gradient in smaller sizes 90% freedom from structural deterioration in >65 y at 12 y in modern designs |
| Pericardial | Carpentier-Edwards Magna Mitroflow (Sorin) | Glutaraldehyde fixed pericardium on titanium stent | Nonthrombogenic Risk of postoperative transvalvular gradient in smaller sizes 90% freedom from structural deterioration in >65 y at 12 y in modern designs | |
| Stentless | Toronto Stentless Porcine Valve Freestyle | Glutaraldehyde fixed pig aortic root | Nonthrombogenic Better hemodynamic profile than stented valves More complex to implant Not designed for use in the mitral position |
The different types of mechanical valve are described according to the shape of the moving part (occluder) that opens and closes the valve orifice (see Table 80–1). Three types are encountered in clinical practice today—ball and cage, tilting disc, and bileaflet valves—the most commonly used mechanical valves.
The first mechanical valves were the ball and cage valves of which the Starr-Edwards valve (Baxter Healthcare Corp. Edwards division, Irvine, CA) (Fig. 80–2) has been used most widely and was still available until recently. The unique features of this design were (1) the fact that the occluder travels completely out of the orifice, reducing the possibility of thrombus or pannus growing from the sewing ring to interfere with the valve mechanism, and (2) the continuously changing points of contact of the ball reduced wear and tear in any one area. Nevertheless, the ball and cage model represented a significant thrombogenic risk, with a linearized risk of thrombotic event of 4% to 6% per year and a recommended International Normalized Ratio (INR) of 2.5 to 3.5 compared with 2.0 to 3.0 for newer tilting disc prostheses.15 Additionally, the high profile of the valve (Fig. 80–3) meant that impingement on the left ventricular wall when implanted in the mitral position, and occasionally left ventricular outflow tract obstruction, could occur. A final consideration was the lack of central blood flow, which was thought to facilitate clot formation (see Fig. 80–3A). Hence, in the late 1960s, efforts focused on designing an alternative that addressed these problems.
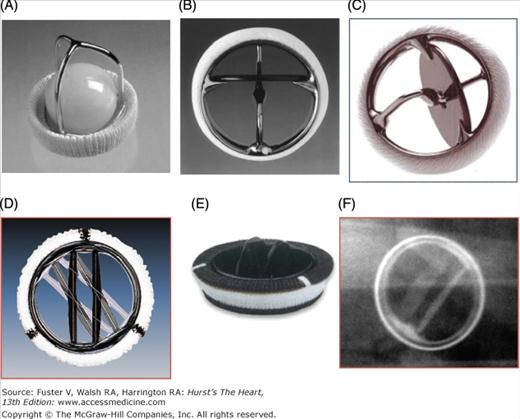
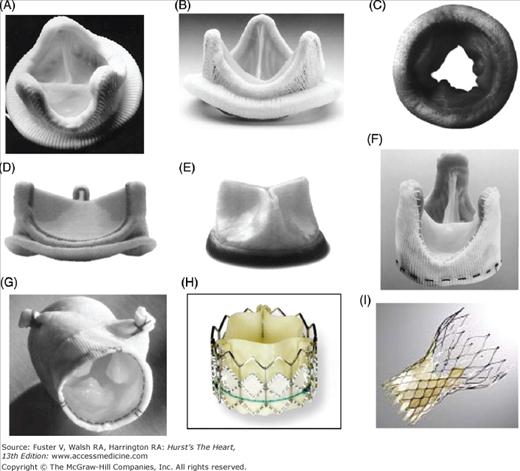
Figure 80–2.
Examples of mechanical prosthetic valves. A. Starr-Edwards ball and cage valve. B. Bjork-Shiley valve tilting disc valve. C. Medtronic Hall tilting disc valve. D. St. Jude Medical bileaflet valve, Masters series. E. CarboMedics bileaflet valve. F. Appearance of CarboMedics prosthesis on plain radiography.
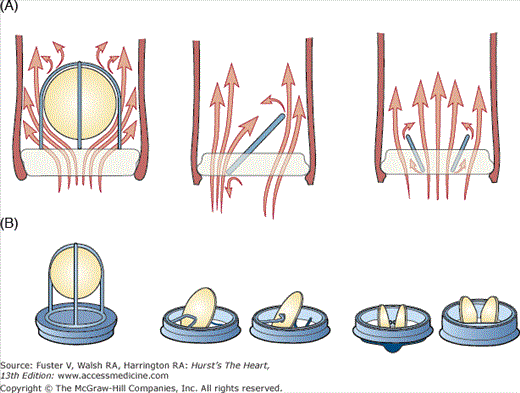
Figure 80–3.
Profile and hemodynamics of each main valve type. A. Whereas ball and cage valves are associated with a lack of central blood ejection fraction, tilting disc valves are associated with turbulent blood flow at the lesser orifice. B. Bileaflet valves have the lowest profile compared with ball and cage valves and tilting disc valves.
Tilting disc prostheses use a single circular leaflet or disc that tilts to occlude or open the valve orifice. They first became available for clinical use in 1969: the Björk-Shiley valve (Shiley, Inc, Irvine, CA) (see Fig. 80–2B) was the first tilting disc valve approved for clinical use in that year. Björk-Shiley valves were successfully implanted in more than 250,000 patients. However, they are no longer produced, primarily because one valve model produced by this company, the Convexo-Concave valve, which represented a slight design modification, displayed a mechanical failure mode in which the outlet strut could fracture allowing the occluder to embolize from its housing. The valve was implanted in 86,000 people worldwide until 1986, and stent fracture was reported in 600 patients up until 1998.16 The Convexo-Concave valves may easily be identified from the serial number, the first two digits of which represent the valve size, the next letter the position (A for aortic and M for mitral), followed by B for Björk-Shiley. A “C” anywhere else in the remaining sequence indicates a Convexo-Concave valve. The risk of strut fracture is not homogenous, and detailed epidemiologic risk modeling now allows accurate risk stratification to determine whether the risk of reoperation to replace the valve is greater or less than the risk of strut fracture for individual patients.16
The advantages of the tilting disc valves over the ball and cage prostheses, including low profile, central blood ejection fraction and lower thrombogenicity, meant that this model became the most popular type of mechanical valve implanted between 1968 and 1980.10 Disadvantages of tilting disc valves include stasis and turbulent flow (see Fig. 80–3A), thrombus and pannus formation interfering with the motion of the disk, and the need for careful orientation of the disc during implantation to avoid outflow obstruction. Another tilting disc valve, the Medtronic-Hall valve (Medtronic), which gained Food and Drug Administration (FDA) approval in 1981, is still in use today. It consists of a pyrolytic carbon-coated disc retained in titanium housing by a strut through a central hole in the disc.
In 1977, valves using two semicircular disks on a central hinge that tilted to open and close the valve orifice (bileaflet valves) were introduced into clinical use by St. Jude Medical. The St. Jude Medical valve (see Fig. 80–2D), approved by the FDA in 1982, has become the most widely used valve prosthesis10 and the basis for models produced by many other manufacturers. The St. Jude Medical valve housing and leaflets are coated with pyrolytic carbon, which is radiolucent. St. Jude Medical produces several models of the basic valve: the HP model uses a smaller sewing ring so that a larger valve can be inserted into small aortic roots, and the Masters model allows the housing to be rotated by the surgeon within the sewing ring after it is implanted to facilitate orientation of the valve, reducing the chance of material such as residual subvalvular apparatus or suture material interfering with the leaflet motion. The Regent model further increases the effective orifice area by allowing supraannular insertion with only the pivot guards and not the housing projecting beyond the aortic annulus. The St. Jude Silzone valve used a silver coated sewing ring with the aim of reducing the ability of bacteria to colonize the valve. Unfortunately, the modified sewing ring was also associated with an increased incidence of paravalvular leak because endothelialization of the valve was inhibited. This model was withdrawn from sale in 2000. A recent study estimated that the excess risk of reoperation of the St. Jude Silzone disappeared by 4 years after implant, and because the minimum time since implant was greater than 5 years, the risk profile of the 28,000 patients still alive with this valve and who have not developed paravalvular leaks is thought to be that of a standard St. Jude Medical valve.17
The St. Jude bileaflet prosthesis demonstrated better effective orifice areas, lower aortic gradients, less aortic insufficiency, lower rates of thromboembolism, and easier insertion than ball and cage and tilting disc valves.18 Since the introduction of the bileaflet valve, more than 2 million bileaflet prostheses have been implanted. The Sulzer CarboMedics valve and ATS Medical valve are the most popular bileaflet valve alternatives.10 The CarboMedics valve, approved by the FDA in 1993, is a bileaflet valve with flat pyrolytic carbon-coated leaflets and a solid carbon housing reinforced with a radiopaque titanium ring (see Fig. 80–2E). The ATS Open Pivot valve, approved in 1995, attempts to reduce thromboembolism formation in the hinge area by locating the hinge socket on the leaflet rather than the housing. The ATS sewing ring is smaller to allow a large valve to be implanted for a given annular diameter without the need for concomitant annular enlargement. The Sulzer CarboMedics valve achieves the same result through a supravalvular implantation. Edwards produced several models of bileaflet prosthesis. The main characteristics of commonly used mechanical prostheses are summarized in Table 80–2.
| Class | AHA/ACC Guidelines | ESC Guidelines |
|---|---|---|
| Class I | 1. A mechanical prosthesis is recommended for AVR in patients with a mechanical valve in the mitral or tricuspid position. (Level of Evidence: C) 2. A bioprosthesis is recommended for AVR in patients of any age who will not take warfarin or who have major medical contraindications to warfarin therapy. (Level of Evidence: C) A bioprosthesis is indicated for MV replacement in a patient who will not take warfarin, is incapable of taking warfarin, or has a clear contraindication to warfarin therapy. (Level of Evidence: C) | Bioprosthesis: Desire of the informed patient. (Level of Evidence: C) Unavailability of good-quality anticoagulation (contraindication or high risk, unwillingness, compliance problems, lifestyle, occupation). (Level of Evidence: C) Reoperation for mechanical valve thrombosis in a patient with proven poor anticoagulant control. (Level of Evidence: C) Mechanical valve: Desire of the informed patient and absence of contraindication for long-term anticoagulation. (Level of Evidence: C) Patient at risk of accelerated SVD. (Level of Evidence: C) Patients already on anticoagulation because of other mechanical prosthesis. (Level of Evidence: C) |
| Class IIa | Patient preference is a reasonable consideration in the selection of aortic valve operation and valve prosthesis. A mechanical prosthesis is reasonable for AVR in patients younger than 65 y who do not have a contraindication to anticoagulation. A bioprosthesis is reasonable for AVR in patients younger than 65 y who elect to receive this valve for lifestyle considerations after detailed discussions of the risks of anticoagulation versus the likelihood that a second AVR may be necessary in the future. (Level of Evidence: C) 2 A bioprosthesis is reasonable for AVR in patients age 65 y or older without risk factors for thromboembolism. (Level of Evidence: C) 3 Aortic valve re-replacement with a homograft is reasonable for patients with active prosthetic valve endocarditis. (Level of Evidence: C)
| Bioprosthesis: Patient for whom future redo surgery would be at low risk Limited life expectancy, severe comorbidity, or age >65-70 y Young woman contemplating pregnancy Mechanical valve: Patients already on anticoagulation because of high risk for thromboembolism. (Level of Evidence: C) Age <65-70 y and long life expectancy. (Level of Evidence: C) Patients for whom future redo surgery would be high risk. (Level of Evidence: C) |
| Class IIb | A bioprosthesis might be considered for AVR in a woman of childbearing age. (Level of Evidence: C) |
Bioprosthetic valves are described according to whether they are made of porcine aortic valves or pericardium (Fig. 80–4). They may be mounted on a metallic stent (stented). Pericardium is usually bovine in origin but may be porcine or equine, and pericardial valves are almost invariably stented. Bioprosthetic valves are also sometimes described according to the method of tissue fixation and treatments used as first-, second-, or third-generation valves. Third-generation valves are expected to offer improved freedom from structural valve degeneration.
Figure 80–4.
Examples of stented and stentless bioprosthetic valve. A. Carpentier-Edwards porcine valve. B. Hancock Modified Orifice II porcine valve. C. Mosaic stented porcine valve. D. Carpentier-Edwards Magna pericardial valve. E. Mitroflow pericardial valve. F. Toronto Stentless Porcine Valve. G. Medtronic Freestyle stentless valve. H. Cribier-Edwards transcatheter pericardial valve. I. CoreValve transcatheter pericardial valve.
All porcine and pericardial valves are now fixed in glutaraldehyde, which, as outlined earlier, was discovered in the 1960s to cross-link collagen fibers and reduce tissue antigenicity, enzymatic degradation, and cell viability.8 Glutaraldehyde fixation of porcine valves was initially performed at normal closing pressure (60-80 mm Hg), which led to some loss of the natural tissue architecture, eventually increasing the predisposition of porcine valves to calcify. To prevent this, zero pressure is now used for porcine prostheses, and low- and zero-pressure fixation is used for pericardial prostheses. Although optimizing fixation methods, the tissue still tends to calcify within 10 to 20 years after implant depending on the patient’s age. The true cause and mechanisms of calcification have been and still are the subject of debate. The general belief is that glutaraldehyde is the culprit, but that does not explain why valves not treated in glutaraldehyde, such as homografts, also calcify, nor why the patient’s own tissues may calcify (eg, calcified aortic valve stenosis or mitral annular calcification).19 In fact, all pathologic tissues tend to calcify under abnormal conditions of stress and biochemical environment with abnormal phosphocalcium metabolism and the absence of living cells. These drawbacks are minimized by glutaraldehyde fixation associated with calcium-mitigating agents using a variety of chemicals, the most effective being surfactant20 and other techniques reducing the amount of phospholipids.21
The first stented glutaraldehyde-preserved porcine valve was homemade and clinically implanted by Carpentier in 1969 and manufactured by Edwards Laboratories in 1970. However, at Carpentier’s request, this valve was made widely commercially available only in 1972 after ensuring that the glutaraldehyde treatment represented a real improvement over his previous method of valve processing. At the same time, Warren Hancock, a former Edwards engineer, founded his own laboratory in the early 1970s to develop a similar stented porcine valve first treated by formalin and then by glutaraldehyde. The Carpentier-Edwards porcine valve bioprosthesis featured a flexible stent and a supraannular configuration (see Fig. 80–4A). Modifications in use include the SupraAnnular valve and the Duraflex low-pressure mitral valve. Hancock subsequently developed a model in which the porcine right coronary cusp was replaced with a leaflet from another porcine valve in an effort to improve the hemodynamic profile of his valve. This Modified Orifice model was released in 1976. The Hancock Modified Orifice II additionally used a thinner stent and a lower profile, making it particularly valuable for insertion in the mitral position, where prominent struts were associated with left ventricular wall rupture. The Biocor stented bioprosthesis valve now marketed by St. Jude Medical is designed for suprannular implantation. Medtronic produces the Mosaic stented bioprosthesis valve (see Fig. 80–4C), which uses a “mosaic of technologies,” including glutaraldehyde fixation anticalcification treatment, zero-pressure fixation, and a flexible stent.
Instead of an intact porcine aortic valve, pericardial valves are manufactured from bovine pericardium and mounted on a stent for ease of implantation. The potential advantages include increased durability through the increased amount of collagen in pericardium and improved hemodynamics resulting from the more symmetrical function of the leaflets. The earliest pericardial valves were the Ionescu-Shiley valves, which had good initial function but high early failure rates.
In 1989, Carpentier developed a unique method of mounting the leaflets on an original stent that did not require stitching into the pericardium itself. This new bioprosthesis, the Carpentier-Edwards Perimount bioprosthesis, benefited from an improved tissue fixation technique, also developed by Carpentier. It gained FDA approval in 1991 and is widely used today in both the earlier version and the more recent Magna valve (which has a narrower sewing cuff and is designed for supraannular implantation) (see Fig. 80–4D). Another pericardial valve, the Mitroflow valve (Sorin Biomedica Cardio srl, Italy) (see Fig. 80–4E), was subsequently developed with a unique design whereby the stent is inside rather than outside the pericardial leaflets, resulting in an increased effective orifice area for a given annular diameter. It is therefore of particular use in the small aortic root.
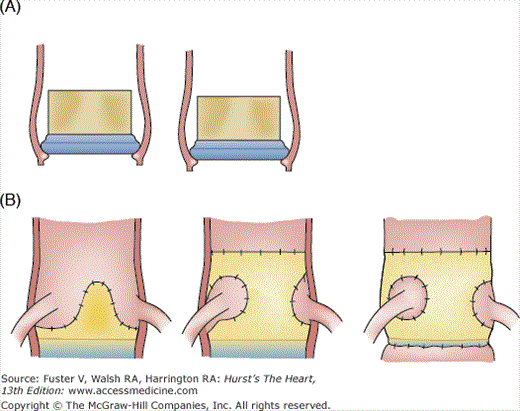
Driven by the desire to further optimize hemodynamic performance by increasing the orifice area, the stents were dispensed with to create stentless valves that use a Dacron cloth or the porcine aortic root as support instead of a metal stent. Two main models of stentless valve are in use today: the Toronto Stentless Porcine Valve (SPV) (see Fig. 80–4F), manufactured by St. Jude Medical, which gained FDA approval in 1995, and the Medtronic Freestlye (see Fig. 80–4G), which gained FDA approval in 1997. The Toronto SPV retains a small portion of the porcine aortic wall and is covered in Dacron cloth to facilitate implantation. The Freestyle consists of the entire porcine aortic root, including the proximal portions of the right and left main stem coronary arteries, with a Dacron support to the sewing ring. This valve may be implanted like a stented valve in the subcoronary position and as a full root replacement (in which only the patient’s native annulus, coronary buttons and mid ascending aorta are retained) or using the inclusion technique (in which a full root replacement is performed but the native aorta is retained as an external buttress) (Fig. 80–5). Root replacement is a useful option when a very small aortic annulus precludes implantation of an appropriately sized valve or in the setting of aortic root pathology, but it is not a routine choice for aortic valve replacement because it is a more complex procedure with longer cardiopulmonary bypass times and greater associated morbidity and mortality in most hands.22 Other stentless valve models include the Baxter Prima Plus, which resembles the Freestyle; the Biocor PSB; and the Cryolife O’Brien. All of these valves are also glutaraldehyde treated.
Figure 80–5.
Methods of aortic valve implantation. A. Subcoronary techniques: supraannular allows a larger valve size for a given annular diameter than intraannular implantation. B. Sketch showing the difference between subcoronary, inclusion, and full root replacement techniques, which may all be performed using the Freestyle stentless porcine valve.
Taking advantage of the unique pliability and softness of glutaraldehyde-treated pericardium, this technology was used by Cribier to develop a valvular bioprosthesis that could be implanted transcutaneously without cardiopulmonary bypass.23 These are valves composed of glutaraldehyde-treated pericardium mounted on metallic stents that are compressible but can be reexpanded when in position, generally the aortic or the pulmonary position. They can be delivered in a retrograde fashion without recourse to cardiopulmonary bypass via a catheter placed in the femoral or axillary artery or antegradely via the apex of the left ventricle. During the few seconds required to expand the stent, the cardiac output is reduced to near zero by rapid ventricular pacing. The native aortic valve is simply displaced by balloon inflation into the coronary sinuses. The options currently available include the Cribier-Edwards valve, now called the Edwards Sapien24 (see Fig. 80–4H), which had been implanted in more than 800 patients by 2008, and the CoreValve (see Fig. 80–4I), now marketed by Medtronic, which had been implanted in more than 2000 patients by the same date.25 The Edwards Sapien valve, designed to be implanted via the transfemoral or the transapical routes, is currently being evaluated in the PARTNER (Placement of AoRTic traNscathetER valves) trial in the United States. In comparison, the CoreValve prosthesis was designed for transfemoral implantation and consists of bovine pericardium mounted on a large self-expanding nitinol stent, which allows left ventricular ejection to continue during stent expansion. Initial results in phase III studies conducted in high-risk patients suggest that both the early learning curve and technologic improvements have impacted outcomes, which appear to be improving.25,26 Although early results do not match those of conventional aortic valve replacement, the highest risk patients in the transcatheter aortic valve replacement series were deemed too high risk for conventional surgery, and the transcatheter groups are therefore not directly comparable to the surgical groups in these nonrandomized studies.27
Outcomes According to Prosthesis Type
A number of studies have compared the clinical outcomes of mechanical valves and bioprostheses, the vast majority of which have been observational studies. In most cases, significant baseline differences in patient demographics, comorbidity, and operative technique make comparison of data very difficult. Grunkemeier et al10 conducted a detailed review of this literature, selecting 165 published series comprising more than 60,000 valves and more than 300,000 valve-years of follow-up to provide a comprehensive overview of outcomes according to prosthesis type. Three randomized trials comparing mechanical with biological prostheses have been carried out: two comparing older valve models in elderly patients in the aortic and mitral position (the Edinburgh Heart Valve Trial, 1975-197928, and the Veterans Affairs Cooperative Study on Valvular Heart disease, 1979-198229). More recently, Stassano et al30 published the results of a randomized trial of bileaflet St. Jude and CarboMedics mechanical valves versus second-generation Carpentier-Edwards bioprosthetic valves in 310 patients aged 55 to 75 years who were operated on from 1995 to 2003 in a bid to compare modern prosthetic valves in a population in which there is some uncertainty as to best practice. There are additionally several multicenter and national registry studies reporting clinical outcome data.31,32 Although published after the majority of the clinical trials, the American College of Cardiology (ACC)/American Heart Association (AHA) Guidelines for Reporting Morbidity and Mortality After Cardiac Valve Interventions presents a useful framework for describing complications after heart valve surgery, as well as definitions of early mortality, long-term survival, structural valve degeneration, nonstructural dysfunction, valve thrombosis, embolism, bleeding events, operated valve endocarditis, reintervention, and all-cause mortality33 (Table 80–3).
| Term | Definition |
|---|---|
| Mortality or operative mortality | Death within 30 days of operation regardless of the patient’s geographic location. Follow-up for 30-day mortality must be complete. Hospital mortality is death within any time interval after operation if the patient is not discharged from the hospital. Hospital-to-hospital transfer is not considered discharge; transfer to a nursing home or rehabilitation unit is considered hospital discharge unless the patient subsequently dies of complications of the operation. |
| Structural valvular deterioration (SVD) | Any change in function (a decrease of one NYHA functional class or more) of an operated valve including: • Operated valve dysfunction or deterioration exclusive of infection or thrombosis Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|