Detection and Quantitation of Wall Motion Abnormalities
Regional left ventricular wall motion and global ventricular function can be analyzed and quantified using a number of schemes. These can be classified as purely qualitative, semiquantitative, and quantitative assessments.
Table 16.2 outlines many of the schemes that are either commonly used today or have been proposed in the past for evaluation of regional wall motion abnormalities. Although detailed quantitative schemes, which measure regional or global function as a percentage of anticipated normal, may be useful for serial studies and investigational protocols, they are not necessary for clinical diagnosis. A compromise that allows semiquantitation and that can be employed easily is the calculation of a wall motion score which is a unitless number directly proportional to the severity and magnitude of wall motion abnormalities.
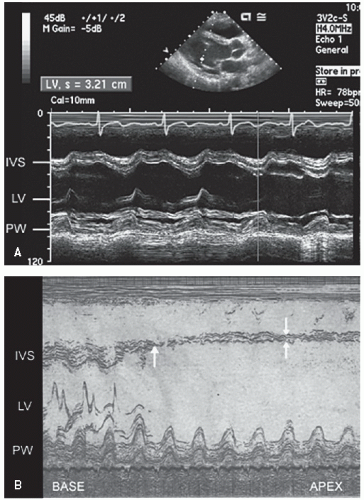
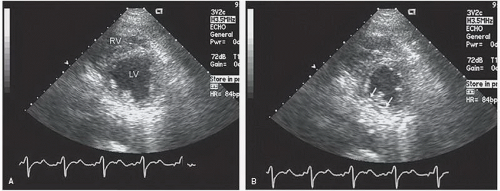
M-mode left ventricular measurements provide only limited information on patients with coronary artery disease, largely
because of the regional nature of the wall motion abnormality (
Fig. 16.12). The linear minor-axis dimension between the posterior left ventricular endocardium and the septum provides an assessment of systolic function at the base of the heart. A twodimensional area measurement of the short axis at the papillary muscle level and the resultant fractional area change may provide a reasonable global assessment of left ventricular function but shares many of the same limitations as M-mode dimensions.
Determination of global ventricular function provides diagnostic and prognostic information in patients with ischemic syndromes. Many of the algorithms for determining global function are discussed in
Chapter 6. The most commonly used assessment of left ventricular systolic function is the ejection fraction. As a matter of convenience, many echocardiographic laboratories give an “eyeball” or visually estimated qualitative assessment of the ejection fraction. Although there are data supporting this approach, it is subjective and highly observer dependent. One can measure left ventricular diastolic and systolic volumes, from which ejection fraction is then calculated. The volumes are frequently indexed to body surface area to allow normalization of data for investigational purposes.
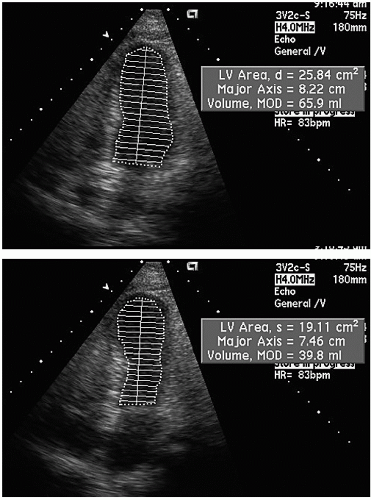
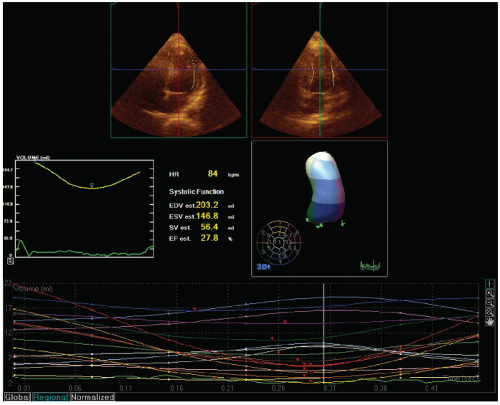
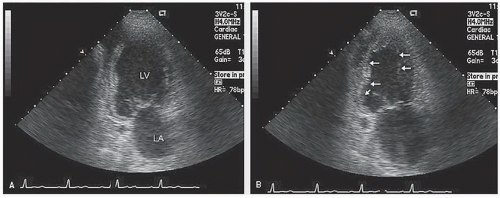
The most commonly used method for determination of left ventricular volume is the Simpson rule, or the rule of disks method. For this method, endocardial borders in diastole and systole are outlined. A series of disks of identical height, each of which corresponds to one of multiple, equally spaced, minor-axis dimensions of the ventricle, are generated. The volume of the individual disks is summed to provide a volume (
Fig. 16.13). If a regional wall motion abnormality is not visualized in the plane of examination, this technique will overestimate the ejection fraction. For this reason, when dealing with patients with coronary disease in whom regional abnormalities are anticipated, biplane methodology is necessary if precise measurements are required. Because of the regional nature of coronary disease, other methods, such as area length calculations, have had less acceptance in evaluating patients with coronary disease.
Evaluation of regional left ventricular function is substantially more complex. There are multiple schemes for regional wall motion assessment (
Table 16.2). The assessment can be undertaken on purely qualitative terms such as an “eyeball” assessment of wall motion as being normal or abnormal or further characterized as hypokinetic, akinetic, or dyskinetic. At the other end of the spectrum, analysis can be undertaken by detailed quantitative schemes in which shortening of multiple endocardial chords around the circumference of the ventricular cavity is calculated.
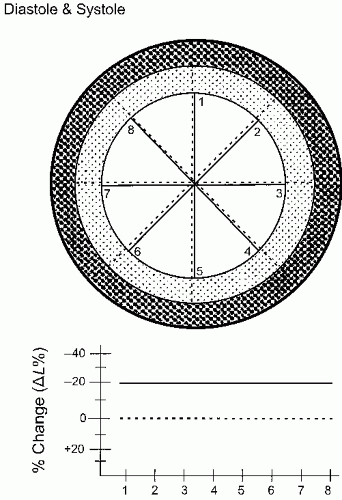
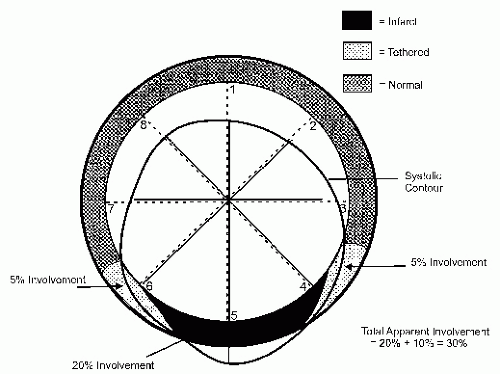
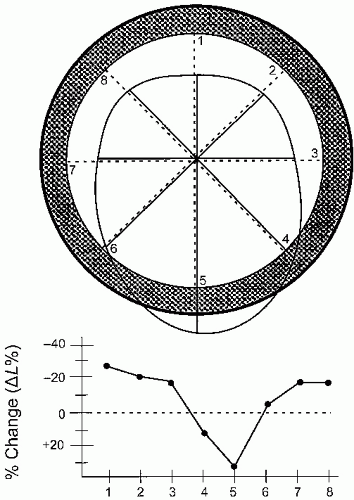
Figure 16.14 schematizes the simplest, quantitative analysis of wall motion using radian shortening and assuming no translational or rotational motion of the heart.
While a number of different detailed, quantitative techniques have been developed and validated in the animal laboratory for quantitation of wall motion abnormalities, the majority of these are not utilized in routine clinical practice. They are limited by the ability to accurately identify endocardial borders and/or myocardial thickening as well as rotational and translational motion and the effects of tethering. As such, while in theory highly accurate for identification of wall motion abnormalities,
they have seen little application in clinical practice. (See
Chapter 6 for a more detailed discussion of quantitative techniques.)
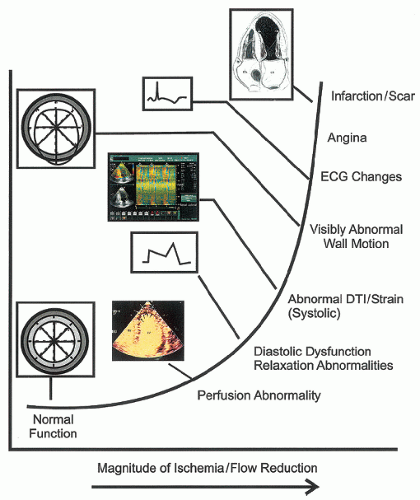
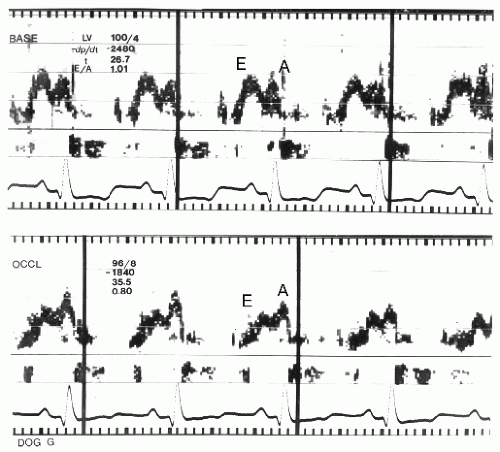
It is important to recognize that normal myocardial motion in systole consists of two closely related events. The first is myocardial thickening during which all layers of the wall contract, resulting in augmentation of the thickness of the myocardium from its normal 8 to 11 mm to 14 to 16 mm. This typically represents a 35% to 40% change in wall thickness. The left ventricular myocardium consists of two layers of myocardial fibers oriented circumferentially around the left ventricle. The contraction of these layers results in both apex to base shortening and circumferential shortening of the left ventricle. The two fiber layers are oriented in opposing directions such that the left ventricle contracts with a wringing motion. When viewed from the apex, the base of the heart rotates clockwise and the apex in a counterclockwise direction. The nature of this wringing motion can be detected with techniques such as Doppler tissue imaging or speckle tracking. While deviation from this normal clockwise-counterclockwise wringing motion has been noted in ischemic heart disease, the incremental benefit of this analysis has not been demonstrated in clinical practice. It should also be pointed out that the apex has limited motion during the ejection and filling phases of the left ventricle. Significant apical motion on an apical view suggests that the transducer is not over the true apex.
Because of the sequence of electrical activation of the heart, not all regions contract at the identical rate or time. In addition to there being substantial temporal and mechanical heterogeneity of contraction in the normal setting, ischemia results in further temporal and mechanical heterogeneity. Although abnormal wall motion is typically described as being akinetic or dyskinetic, detailed analysis of the sequence of contraction often reveals temporal variations of these contraction abnormalities. One such variation is early systolic contraction followed by dyskinetic motion rather than dyskinesis throughout the entire duration of systole. A second is marked delay in onset of contraction but with nearly normal excursion (tardokinesis). The implications of these latter two wall motion abnormalities vary with the clinical setting. Either can be seen as a normal variant, as a manifestation of ischemia, or in the postischemia period. As a general rule, if the wall motion abnormality is very brief (<50 milliseconds), it is more likely to be a normal variant than a manifestation of myocardial ischemia.
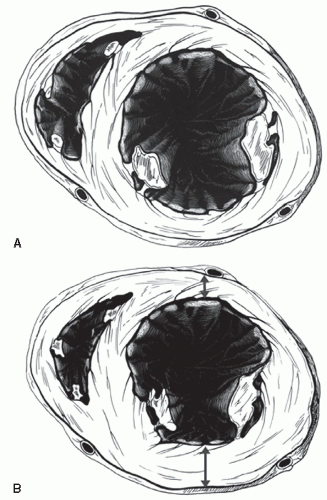
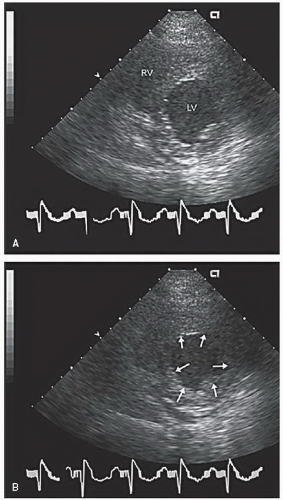
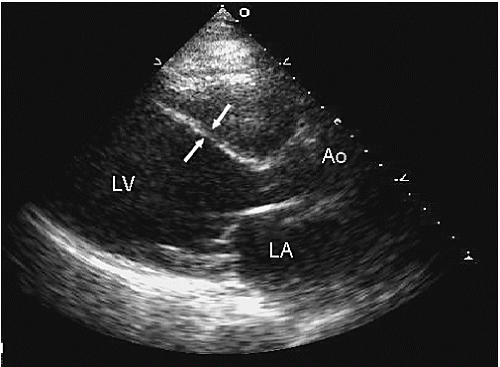
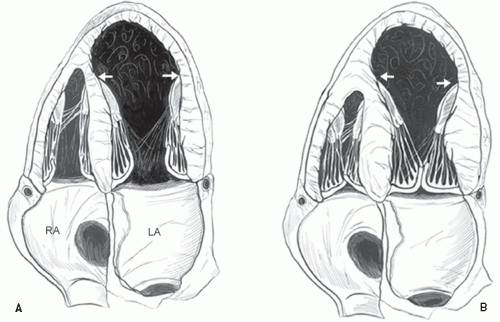
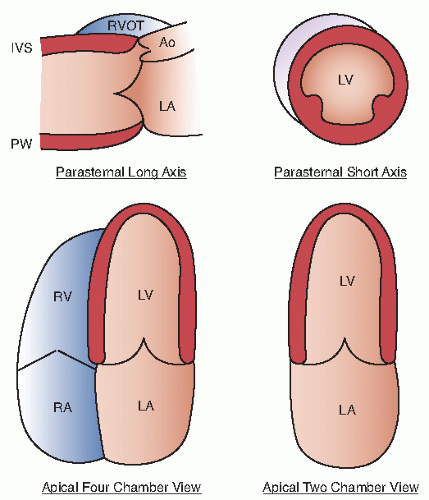
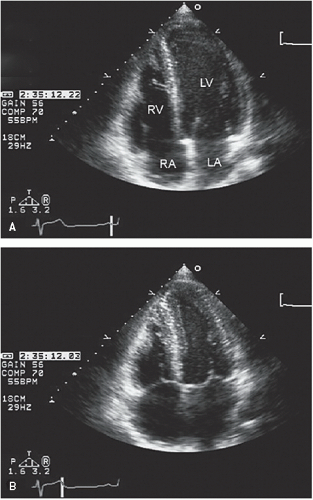
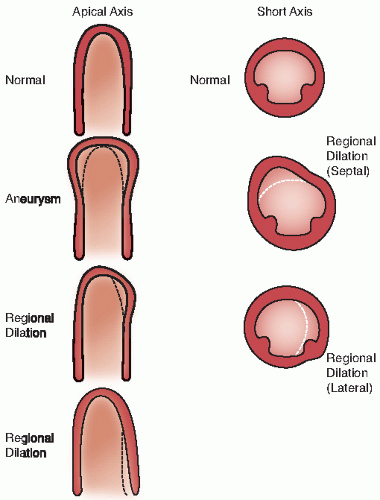
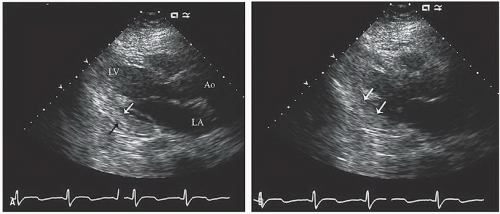
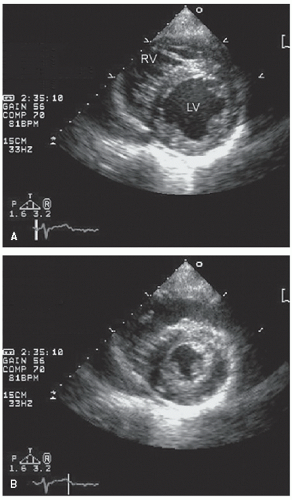
An additional qualitative indicator of abnormal ventricular function involves assessment of ventricular geometry. The normal left ventricle is best described as a cylinder with an apical cone resulting in “bullet”-shaped geometry. This bullet-shaped geometry is noted in the apical four- and two-chamber views as well as in the subcostal view. In the short-axis view, normal left ventricular geometry is circular. In the parasternal long-axis view, normal geometry involves a slight concave curvature of both the ventricular septum and the inferoposterior wall, with the direction of concavity for each wall pointing toward the center of the ventricle. Normal geometry is schematized in
Figure 16.15 and further illustrated in
Figures 16.16 and
16.17. Abnormal geometry is often most apparent in the apical
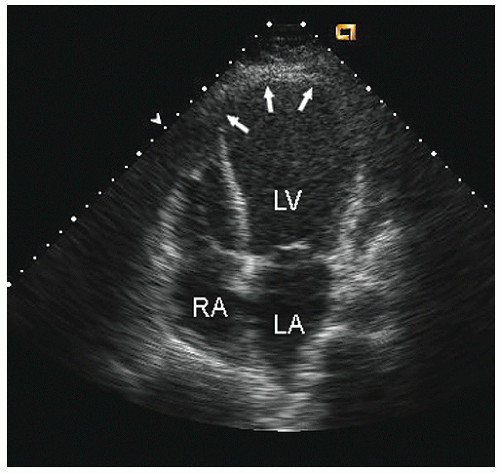
four-chamber view and may involve rounding of the apex or asymmetry of apical shape as opposed to smooth bulletlike tapering (
Fig. 16.18). When evaluating an echocardiogram for an ischemic wall motion abnormality, it is important to quickly assess the left ventricular geometry because it often provides a very rapid clue to the presence of abnormal regional function.
When dealing with coronary disease, it is imperative to adopt a regional approach to the description of wall motion abnormalities, whether that description is a highly detailed quantitative scheme or a simple “eyeball” approach.
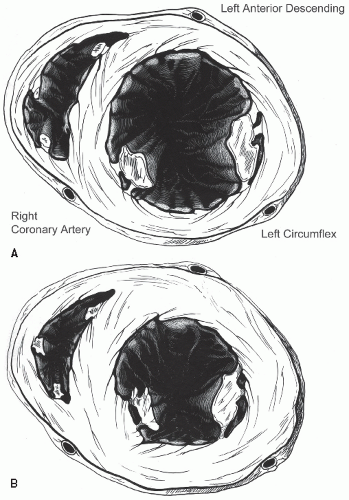
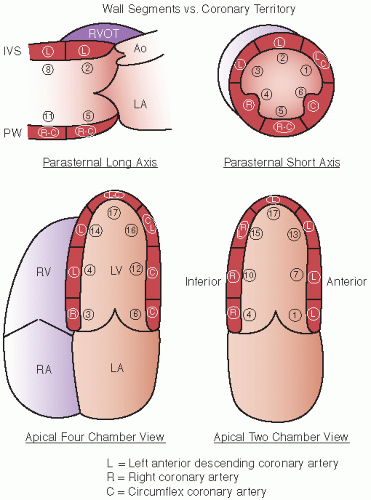
Figure 16.19 schematizes the standard segments of the left ventricle that are commonly employed for analysis as well as the coronary arteries that usually perfuse those segments. Previous schemes employed a 16-segment model. More recently, a 17-segment approach has been recommended in which the 17th segment represents the true apex. This approach allows a more precise correlation with the segments visualized and analyzed by competing imaging techniques. The new segmentation schematic renames the segments, dropping the term “posterior” (
Table 16.3). In general, the anterior septum and anterior wall are perfused by the left anterior descending coronary artery and its branches, and the inferior wall in the area of the posterior interventricular groove by the right coronary artery.
Figure 16.19 outlines the most prevalent distribution of coronary arteries to the various segments. There can be substantial overlap in the inferior, lateral, and anterolateral segments, depending on the dominance of the right and left circumflex coronary arteries. The inferoapical segment represents an overlap zone between the distal left anterior descending coronary artery and the distal right coronary artery, and the apical lateral wall represents an overlap between the circumflex and the left anterior descending coronary arteries. This type of scheme that attributes the coronary artery territories to different regions can be superimposed on any of the semiquantitative or quantitative schemes to assist in linking regional wall motion abnormalities to the coronary artery responsible for wall motion abnormality.
The simplest assessment of wall motion consists of description of wall motion as being normal or abnormal, typically further characterized as hypokinetic, akinetic, and dyskinetic in each region of the myocardium. This assessment suffices for the immediate detection of an ischemic event but does not provide information that can be readily communicated with
respect to the size of myocardial infarction or the size of an area in jeopardy.
The next level of complexity for quantitation of wall motion abnormalities involves generation of a wall motion score or score index. This methodology involves describing the wall motion characteristics of each of the predefined segments as being normal, hypokinetic, akinetic, dyskinetic, or aneurysmal. A numerical score, typically 1 to 5, is then applied to each of these segments (
Table 16.4), and the total score is divided by the number of segments evaluated to create a wall motion score index. A ventricle with completely normal wall motion has a wall motion score index of 1.0 (total score divided by the number of segments), with higher scores representing progressively greater degrees of ventricular dysfunction. This global score, representing overall left ventricular wall motion, can then be subdivided into an anterior score, representing the distribution of the left anterior descending coronary artery, and a posterior score, representing the right plus circumflex coronary artery territories. Often, because of the tremendous overlap in the posterior circulation, an effort is not made to separate the independent contribution of the right coronary artery and the circumflex coronary artery. It is often helpful to also calculate the percentage of segments with normal motion.
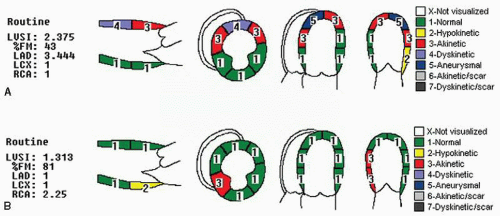
Figure 16.20 presents examples of wall motion score indexes. In
Figure 16.20A, note that the global score of 2.375 is made up entirely of a wall motion abnormality in the left anterior descending coronary territory, whereas the posterior territories are normal.
Additional modifications of the wall motion score index have included an additional descriptive score for scar. Typically, the number assigned for scar is used only for descriptive purposes and the numeric value corresponding to the wall motion abnormality (i.e., 2, 3, or 4) is used for calculation purposes. For example, an akinetic scarred segment will receive a descriptive value of 6, but when calculating the wall motion score index, it is given a value of 3 because it is akinetic. Although allowing for the description of the scar and its extent, it avoids attributing a greater functional deficit to a segment than is actually present.
Other modifications have included using a score of 0 for hyperdynamic. As with the aneurysm score, this allows description of walls with compensatory hyperkinesis; however, it may result in relative underestimation of the deficit attributable to the infarct because the global numeric score now allows the compensatory hyperkinesis to reduce the impact of the wall motion abnormality. By using a score of 1.0 for calculation purposes, the regional wall motion score will remain abnormal even if overall left ventricular function is normal due to compensatory hyperkinesis. Further modifications of a wall motion score scheme have included intermediate scores of 1.5 and 2.5 for mild and severe hypokinesis, respectively, which provide additional quantitative information when evaluating patients during cardiovascular stress or in following recovery of function after myocardial infarction.
Role of the Three-dimensional Echocardiography
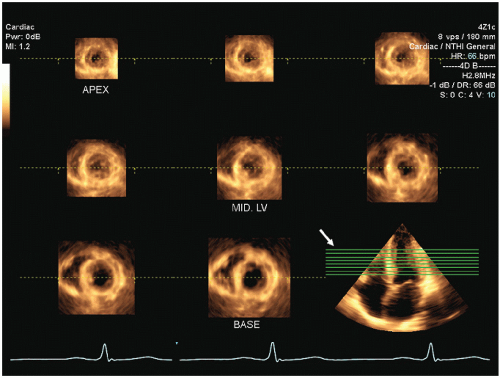
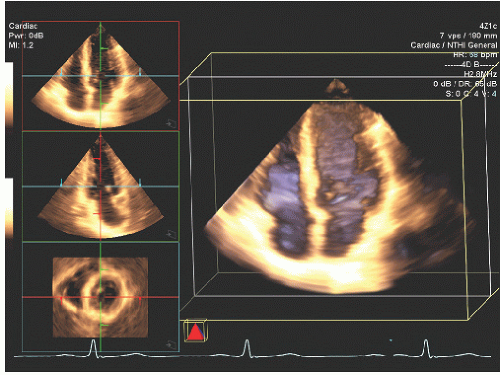
Three-dimensional echocardiography potentially provides an incremental method for evaluating left ventricular wall motion and extraction of detailed parameters of left ventricular function. One clinically relevant application of three-dimensional echocardiography relies on an automated or semiautomated extraction of the left ventricular border from which a “shell” model of the left ventricular volume can be created (
Fig. 16.21). Carefully done clinical studies have demonstrated the superiority of left ventricular volumes determined from three-dimensional echocardiography with respect to absolute accuracy and reproducibility. The three-dimensional volume can be automatically divided into subvolumes corresponding to either a 16- or a 17- segment model of regional wall motion, analogous to that used
for generation of a wall motion score. In theory, this method for analysis of regional ventricle function should provide information equivalent to that from visual analysis of left ventricular wall motion. In reality, technical parameters, such as dropout of the endocardial border and deficiencies in the algorithms used to identify precise boundaries, may reduce the actual impact of this technology in clinical practice. Multiple two-dimensional image planes can be extracted from a threedimensional data set allowing simultaneous visualization of a wall motion abnormality from two or more imaging perspectives (
Figs. 16.17 and
16.22). While technically feasible, realtime or reconstructed images from three-dimensional data sets remain limited by frame rate, and image quality generally is not equivalent to that obtained from dedicated two-dimensional transducers.
Doppler Tissue Imaging and Speckle Tracking
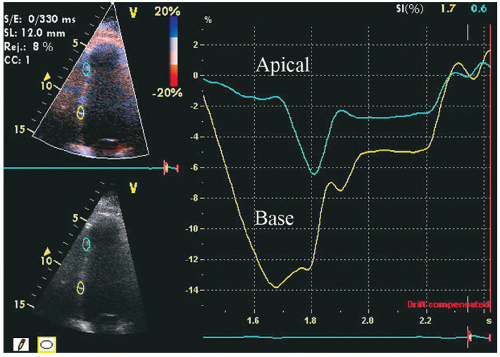
The most recent approach to analysis of regional wall motion has been with either Doppler tissue imaging or “speckle” tissue tracking. These highly sophisticated techniques allow tracking of wall motion in one or more regions of interest, or along a predefined length of the myocardium, from which myocardial deformation can be determined. In its simplest form, this provides analysis of the velocity of myocardial motion at a single point from which displacement can be calculated. At the next step of complexity two adjacent points can be compared for their location and velocity. From this comparison, strain, representing the degree to which the two regions of interest either move toward or away from each other (
Fig. 16.23), or strain rate, representing the velocity of the change in length of the predefined segment, can be determined. Experimental data suggest that both strain and strain-rate imaging are more sensitive and earlier markers of myocardial dysfunction than is visual analysis of wall motion.
The basic techniques for determining strain and strain rate were discussed in
Chapter 3 and further in
Chapter 6 dealing with evaluation of left ventricular function. From a clinical perspective, the clinician should be cognizant of the fact that the algorithms for determining strain and strain rate are highly technique dependent and absolute values of normal vary with location in the myocardium and from patient to patient, making analysis of a subtle deviation from “normal” at any single timepoint problematic. Scrupulous attention to detail is essential with respect to placement of regions of interest to provide data equivalent to that seen in the experimental setting. In view of the complexities of obtaining noise-free strain and (especially) strain-rate signals, this technique is infrequently employed in routine clinical practice. It has shown some promise in stress echocardiography where serial changes are tracked in predefined regions in a given patient. As discussed earlier, the normal wringing contraction motion of the left ventricle is related to the opposing direction of contraction of endocardial and epicardial fibers. Selective ischemia of one layer (typically the endocardial) will alter the normal ventricular torsion and may be a specific marker of selective subendocardial ischemia.
Other Methods for Evaluating Ischemic Myocardium
There are several other technologies that can be brought to bear in evaluating patients with acute ischemic syndromes. Tissue characterization has shown promise for providing incremental information regarding myocardial contractility. This technique relies on evaluating the cyclic variation in backscatter (returning signals from the myocardium). In the absence of myocardial ischemia, the overall intensity of returning signals within the myocardium varies phasically with the cardiac cycle. The presence of even mild myocardial ischemia results in a reduction in this cyclic variation of intensity.
Contrast echocardiography using new perfluorocarbon- or nitrogen-based agents has shown promise for evaluating the integrity of capillary level flow in the myocardium. Myocardial contrast echocardiography can be used for detection of coronary stenosis. Demonstration of preserved microvascular perfusion with myocardial contrast echocardiography has correlated with myocardial viability and subsequent recovery of function in both experimental and clinical myocardial infarction. This topic was discussed in
Chapter 4.


 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access