Ventricular Tachycardia
Nitish Badhwar, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Nonsustained: three or more consecutive QRS complexes of uniform configuration of ventricular origin at a rate of more than 100 bpm.
Nonsustained: three or more consecutive QRS complexes of uniform configuration of ventricular origin at a rate of more than 100 bpm.
![]() Sustained: lasts more than 30 seconds; requires intervention for termination.
Sustained: lasts more than 30 seconds; requires intervention for termination.
![]() Monomorphic ventricular tachycardia.
Monomorphic ventricular tachycardia.
![]() Polymorphic ventricular tachycardia: beat-to-beat variation in QRS configuration.
Polymorphic ventricular tachycardia: beat-to-beat variation in QRS configuration.
The magnitude of ventricular tachycardia (VT), one of the most common health problems encountered in clinical practice, can best be appreciated in terms of its various clinical manifestations, which include ventricular fibrillation (sudden cardiac death [SCD]), syncope or near syncope, and wide QRS tachycardia.
The most serious is its degeneration into ventricular fibrillation, producing cardiac arrest and SCD that accounts for 200,000 deaths a year. The second most serious clinical presentation is syncope. Although the overall prevalence of VT-related syncope is unclear, it is estimated to be frequent because inducible VT (via electrical stimulation) is the most common arrhythmia detected in patients with unexplained syncope. A high prevalence of SCD (> 20% incidence within the ensuing 12 months) is noted in patients with syncope from cardiovascular causes, suggesting that undiagnosed VT may be an underlying cause of sudden death in patients with unexplained syncope. The third most significant clinical manifestation of VT is a wide QRS complex tachycardia that is often hemodynamically well tolerated.
DIAGNOSTIC ISSUES
1. Underdiagnosis
VT as a cause of morbidity and mortality is grossly under-diagnosed, potentially leading to mismanagement. This may be particularly true when the clinical presentation is unexplained syncope because no concomitant electrocardiographic (ECG) documentation is available. In the case of cardiac arrest or SCD, acute myocardial infarction rather than an arrhythmic problem is often assumed to be responsible. Most persons who have suffered sudden death have no evidence of acute myocardial necrosis, even though the episode often occurs in patients with underlying coronary artery disease. Managing the underlying coronary artery disease with no regard to treating the concomitant VT is inadequate.
2. Misdiagnosis
When hemodynamically stable VT is recorded on the surface ECG, it is often misdiagnosed as supraventricular tachycardia (SVT) with aberrant conduction. Any subsequent management is therefore directed toward SVT. Although the exact logic for this line of thinking is unclear, the main reason may be that the hemodynamic stability is associated with the broad QRS rhythm and thus the erroneous belief that the problem cannot be VT.
The clinical presentation of VT depends on many factors, including rate, ventricular function, presence of concomitant coronary artery disease, the presence or absence of cardioactive drugs, and even the patient’s posture at the time of onset. Hemodynamic tolerance of VT can, therefore, vary considerably in different situations; at times, it can vary in the same patient, and it is prudent not to exclude the diagnosis of VT on the basis of hemodynamic tolerance alone. It must be understood that approximately 80% of the patients with sustained wide QRS tachycardia have VT. To avoid misdiagnosis, the clinician can either use the established ECG criteria (discussed in the next section) that distinguishes VT from SVT with aberrant conduction or simply assume the presence of VT. The assumption of VT is more often correct; it is also safer because misdiagnosing VT as SVT is a riskier judgment error than vice versa.
3. Diagnostic Approach to the Patient with Wide QRS Complex Tachycardia
The diagnosis of wide QRS complex tachycardia by ECG analysis has always been a challenge for clinicians. The differential diagnosis includes VT, SVT with aberrant conduction, and preexcited tachycardia in patients with Wolff-Parkinson-White (WPW) syndrome. Figure 13–1 depicts, schematically, the reasons for normal and broad QRS complexes. Preexcited tachycardia results from antegrade activation of the ventricle via an accessory pathway in patients with WPW syndrome, which can present with atrial fibrillation, atrial flutter, atrial tachycardia, atrioventricular nodal reentry tachycardia (AVNRT), or antidromic tachycardia (Figure 13–2). Preexcited tachycardia is a rare cause of wide QRS complex tachycardia (5–8% of cases); however, the QRS pattern of preexcited QRS complex can be difficult to distinguish from VT because in both instances the QRS starts with muscle-to-muscle conduction. ECG artifact can also mimic wide QRS complex tachycardia and be misdiagnosed as VT, leading to expensive testing and even placement of implantable cardioverter-defibrillator (ICD). Clues to the diagnosis include absence of hemodynamic deterioration, an unstable baseline, association with body movement, and ability to march the normal QRS complexes through the artifact (“notches sign”) at sinus R-R interval (Figure 13–3). A number of surface ECG criteria, including the atrioventricular (AV) relationship, the QRS complex duration, specific QRS morphology, and the QRS complex axis, have been established to distinguish VT from SVT with aberrant conduction. These criteria are helpful in arriving at an accurate diagnosis if they are used in a systemic fashion.
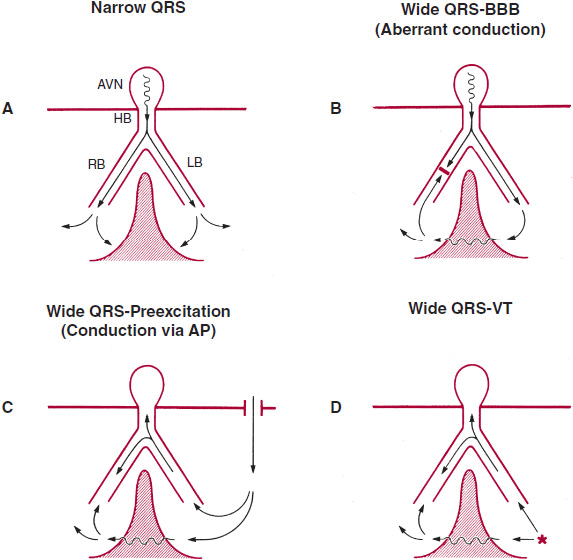
![]() Figure 13–1. Mechanism of wide QRS. A: Narrow QRS from simultaneous activation of the right and left ventricles. In the three types of wide QRS shown in B–D, there is sequential rather than simultaneous activation of the left and right ventricle and a variable amount of muscle-to-muscle conduction. AP, accessory pathway; AVN, atrioventricular node; BBB, bundle branch block; HB, His bundle; LB, left bundle; RB, right bundle. (Reproduced, with permission, from Akhtar M, et al. Electrophysiological spectrum of wide QRS complex tachycardia. In: Zipes DP, et al., eds. Cardiac Electrophysiology. From Cell to Bedside. Philadelphia: WB Saunders; 1990.)
Figure 13–1. Mechanism of wide QRS. A: Narrow QRS from simultaneous activation of the right and left ventricles. In the three types of wide QRS shown in B–D, there is sequential rather than simultaneous activation of the left and right ventricle and a variable amount of muscle-to-muscle conduction. AP, accessory pathway; AVN, atrioventricular node; BBB, bundle branch block; HB, His bundle; LB, left bundle; RB, right bundle. (Reproduced, with permission, from Akhtar M, et al. Electrophysiological spectrum of wide QRS complex tachycardia. In: Zipes DP, et al., eds. Cardiac Electrophysiology. From Cell to Bedside. Philadelphia: WB Saunders; 1990.)
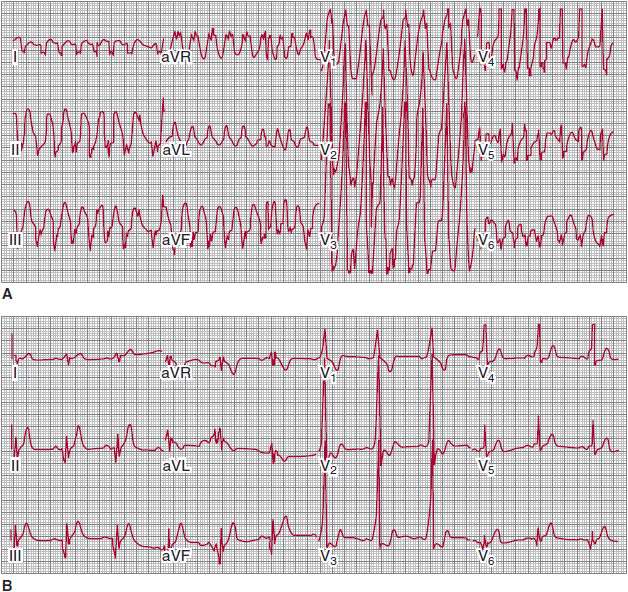
![]() Figure 13–2. A: Preexcited wide complex tachycardia in a patient with Wolff-Parkinson-White (WPW) and atrial fibrillation B: Sinus rhythm electrocardiogram showing short PR interval and delta wave consistent with left posterior WPW. (Reproduced, with permission, from Turakhia MP, et al. Indian Pacing Electrophysiol J. 2009;9[2]:130–3.)
Figure 13–2. A: Preexcited wide complex tachycardia in a patient with Wolff-Parkinson-White (WPW) and atrial fibrillation B: Sinus rhythm electrocardiogram showing short PR interval and delta wave consistent with left posterior WPW. (Reproduced, with permission, from Turakhia MP, et al. Indian Pacing Electrophysiol J. 2009;9[2]:130–3.)
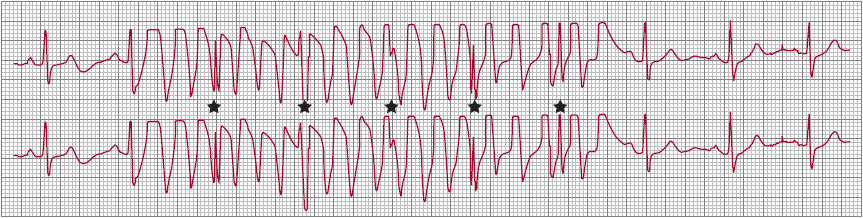
![]() Figure 13–3. Artifact mimicking ventricular tachycardia. The QRS complexes are seen as notches that are marching at the regular sinus interval (*). (Reproduced, with permission, from Badhwar N, et al. J Intensive Care Med. 2012;27[5]:267–89.)
Figure 13–3. Artifact mimicking ventricular tachycardia. The QRS complexes are seen as notches that are marching at the regular sinus interval (*). (Reproduced, with permission, from Badhwar N, et al. J Intensive Care Med. 2012;27[5]:267–89.)
 Atrioventricular Relationship
Atrioventricular Relationship
In SVT, the arrhythmia arises in the atria or AV junction and reaches the ventricles through the AV node and His-Purkinje system. Because the atrial arrhythmia is the primary event, either a 1:1 AV response or a varying degree of AV block occurs, but in either case, the atrial rates equal or exceed ventricular rates. During VT, a retrograde block often leads to either AV dissociation or a varying degree of ventriculoatrial conduction ratios, but the ventricular rates equal or exceed the atrial rate. When AV dissociation can be recognized, it is the most reliable criterion for VT (Figures 13–4 and 13–5). This criterion lacks sensitivity, however, because the P waves can be identified on the surface ECG in only 25% of patients with VT. In patients with slower VT and AV dissociation, intermittent ventricular capture can result in fusion with narrow QRS complexes during wide QRS complex tachycardia. This useful but rarely observed finding is also 100% specific for the diagnosis of VT.
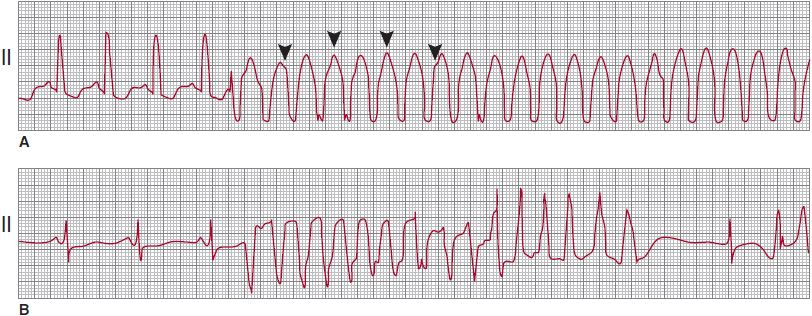
![]() Figure 13–4. A: Monomorphic ventricular tachycardia (VT), with a uniform QRS appearance for all complexes. Arrowheads indicate superimposed P waves. B: Polymorphic VT, with a beat-to-beat variation in the QRS morphology; QT-interval prolongation follows the termination of the VT episode. (Reproduced, with permission, from Akhtar M. Circulation. 1990;82:1561.)
Figure 13–4. A: Monomorphic ventricular tachycardia (VT), with a uniform QRS appearance for all complexes. Arrowheads indicate superimposed P waves. B: Polymorphic VT, with a beat-to-beat variation in the QRS morphology; QT-interval prolongation follows the termination of the VT episode. (Reproduced, with permission, from Akhtar M. Circulation. 1990;82:1561.)
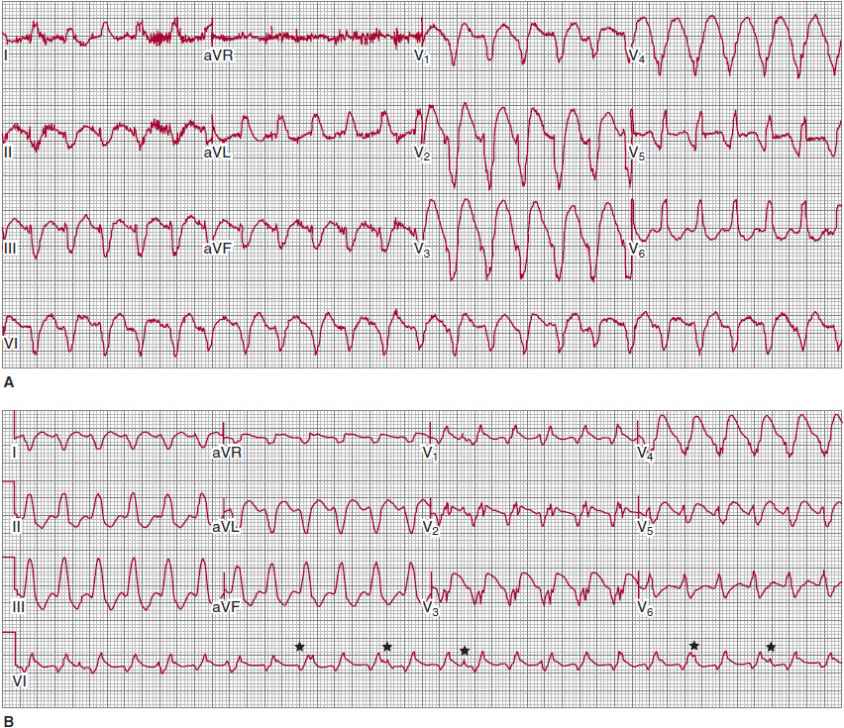
![]() Figure 13–5. A: Scar-related ventricular tachycardia (VT), with a left bundle branch block left axis morphology in a patient with ischemic cardiomyopathy and previous myocardial infarction. B: Right bundle branch block right axis morphology VT in the same patient at the same rate suggesting that both forms of VT have the same circuit (that revolves around the mitral annulus) with different exits causing the difference in morphology. Atrioventricular dissociation is noted in the rhythm strip on V1 (*) that is 100% specific for VT.
Figure 13–5. A: Scar-related ventricular tachycardia (VT), with a left bundle branch block left axis morphology in a patient with ischemic cardiomyopathy and previous myocardial infarction. B: Right bundle branch block right axis morphology VT in the same patient at the same rate suggesting that both forms of VT have the same circuit (that revolves around the mitral annulus) with different exits causing the difference in morphology. Atrioventricular dissociation is noted in the rhythm strip on V1 (*) that is 100% specific for VT.
 QRS Complex Duration
QRS Complex Duration
For the reasons listed earlier, the QRS complex duration is the widest in VT and narrowest in aberrant conduction. To distinguish VT from SVT with aberrant conduction on the basis of QRS duration alone, however, some specific aspects must be considered. In the absence of cardioactive drugs and extensive myocardial fibrosis, aberrancy rarely results in a QRS duration of more than 140 ms with a right bundle branch block (RBBB) pattern (see Figure 13–5) or more than 160 ms with a left bundle branch block (LBBB) configuration. In the presence of intramyocardial conduction delay from drugs (such as class I antiarrhythmic agents) and myocardial fibrosis, the QRS width may also exceed these values in SVT with aberrant conduction. Conversely, on a rare occasion, VT can present as a narrow QRS tachycardia (< 120 ms that is narrower than the conducted QRS complex) when there is near-simultaneous activation of the two ventricles, perhaps from the septum.
 Specific QRS Morphology
Specific QRS Morphology
The prevalence of LBBB versus RBBB morphology among the causes of wide QRS is comparable in both VT and SVT with aberrant conduction; it is therefore of no diagnostic value. The typical RBBB is a triphasic complex best seen in V1 as rsR’ or rSR’ pattern and in lead I as qRs, qRS pattern. Similarly, a typical LBBB has no initial q wave in lead I and a small r and a rapid S wave in V1. Because of the myocardial origin of most forms of VT, however, the QRS appearance is not exactly like a typical LBBB or RBBB. Many ECG criteria, therefore, have exploited this difference to separate VT from aberrant conduction. A study that analyzed the morphology of premature ventricular complexes and aberrantly conducted beats of RBBB morphology in V1 found that the triphasic RsR’ pattern with R’ > R was predominant in aberrant conduction (70%) compared with premature ventricular complexes (6%). Monophasic pattern or R > R’ was seen in the premature ventricular complex beats. The limitation of that study is that origin of the anomalous beats (SVT versus VT) was also based on the ECG (presence or absence of preceding P wave). A retrospective ECG analysis of 70 patients with SVT and 70 patients with VT in whom His bundle recordings were used to determine the site of origin of the wide QRS complex tachycardia found that VT was favored by monophasic or biphasic R waves in V1 and an R:S ratio less than 1 in V6 in patients with RBBB morphology and any Q wave in V6 in patients with LBBB morphology. A study of 150 consecutive wide QRS complex tachycardia cases found that 12-lead QRS morphology during wide QRS complex tachycardia was different from that during preexisting bundle branch block in sinus rhythm, favoring a diagnosis of VT. The investigators also noted that a positive QRS concordance (positive complexes V1–V6) is uncommon in aberrancy, but a negative QRS concordance can occur during aberrant conduction in a small percentage of cases. Another study that evaluated wide QRS complex tachycardia with LBBB morphology in V1 found that an R wave of > 30 ms, notching in the down stroke of the S wave and an RS (beginning of QRS complex to nadir of S wave) interval > 60 ms in V1 or V2, and any Q wave in V6 favored a diagnosis of VT. In a prospective analysis of wide QRS complex tachycardia (with RBBB and LBBB morphology), the following two criteria for a diagnosis of VT were proposed: (1) absence of R-S in all precordial leads, and (2) R-S interval > 100 ms (measured from the beginning of the QRS complex to the nadir of the S wave) in any precordial lead. Finally, the presence of QR complex in any lead during wide QRS complex tachycardia also favors a diagnosis of VT.
 QRS Complex Axis
QRS Complex Axis
The axis orientation on a 12-lead ECG ranging from normal (-30° to +90°), left (-31° to -90°), or right (+91° to +180°) has significant overlap across the causes of wide QRS complex tachycardia and is of little diagnostic value. The axis range of -91° to 180°, however, is usually not seen in aberrant conduction. The axis location of this extreme during SVT is, therefore, unlikely unless there was a nonarrhythmic reason for it, such as severe right ventricular hypertrophy or lung disease. Similarly, a combination of right axis with LBBB pattern is almost always seen in patients with VT. A previous history of myocardial infarction (MI) and an axis change of more than 40° between sinus rhythm and wide QRS complex tachycardia may independently favor VT over SVT.
 History, Physical Examination, and 12-Lead ECG
History, Physical Examination, and 12-Lead ECG
A detailed history and physical examination can provide clues to the diagnosis of wide QRS complex tachycardia. A history of MI favors VT as the diagnosis of wide QRS complex tachycardia. The presence of irregular cannon waves and variable intensity of S1 suggest AV dissociation and are indicative of VT. Carotid massage (performed carefully after ruling out a bruit) that leads to termination of wide QRS complex tachycardia suggests SVT as the mechanism of the arrhythmia (an exception is idiopathic VT arising from the right ventricular outflow tract).
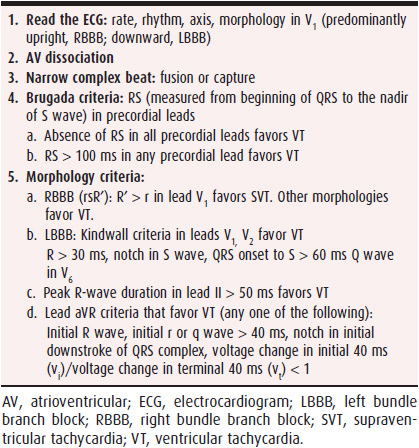
An old ECG in sinus rhythm showing Q waves indicates VT; SVT is the diagnosis if the old ECG shows bundle branch block pattern that matches the 12-lead ECG during wide QRS complex tachycardia; and preexcited tachycardia is inferred from the ECG showing WPW pattern that is similar to wide QRS complex tachycardia ECG pattern. It is essential to obtain a 12-lead ECG during wide QRS complex tachycardia to compare it to the sinus rhythm ECG and look for subtle findings like AV dissociation and narrow beats (fusion and capture) that might not be evident in some leads. Table 13–1 outlines an approach to diagnosing wide QRS complex tachycardia by analyzing the ECG. The first step is to read the ECG with emphasis on rate, regularity (atrial fibrillation with preexcited tachycardia is irregular), axis (extreme northwest axis suggests VT), and morphology in V1. The next step is looking for AV dissociation (V > A) that is facilitated by marching the sinus P waves at the onset and termination of the wide QRS complex tachycardia if available. Narrow QRS complexes in a wide QRS complex tachycardia (caused by capture and fusion of conducted beats through the AV node-His-Purkinje system) are also 100% specific for VT. The next step is to use the Brugada criteria by evaluating the R-S complexes in precordial leads. Absence of R-S complex in all precordial leads or a R-S greater than 100 ms in one precordial lead favor a diagnosis of VT. The last step is using the morphology criteria to differentiate VT from SVT. Morphology criteria for RBBB and LBBB in leads V1, V2, and V6 have been discussed earlier. The onset of QRS complex to peak of the R wave or nadir of S wave in lead II > 50 ms favors a diagnosis of VT. New criteria based only on analysis of lead avR during tachycardia have been proposed to differentiate SVT from VT. Criteria in avR that favor VT include presence of initial R wave, initial r or q wave > 40 ms, notch in initial downstroke of QRS complex, and voltage change in initial 40 ms (vi)/voltage change in terminal 40 ms (vt) < 1. The morphology criteria have certain limitations because idiopathic VT (fascicular VT, outflow tract VT) and bundle branch reentrant VT can have a typical bundle branch block pattern on the ECG and can be misdiagnosed as SVT with aberrancy, whereas some patients with SVT who take antiarrhythmic medications can have atypical bundle branch block pattern on the ECG, leading to a misdiagnosis of VT.
Table 13–1. Approach to the ECG with Wide QRS Complex Tachycardia
Vereckei A, et al. New algorithm using only lead aVR for differential diagnosis of wide QRS complex tachycardia. Heart Rhythm. 2008;5(1):89–98. [PMID: 18180024]
CLASSIFICATIONS OF VENTRICULAR TACHYCARDIA
The spectrum of VT in Table 13–2 lists the two most common varieties encountered in clinical practice and their underlying causes. This classification helps approach the diagnosis in a systematic fashion and directs the physician to specific therapeutic options.
Table 13–2. Classification and Causes of Common Ventricular Tachycardias
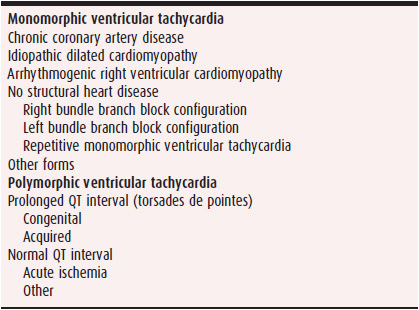
Monomorphic VT is a form of VT in which the QRS complex configuration is uniform from beat to beat in all the surface ECG leads (see Figure 13–4A). In polymorphic VT, a beat-to-beat variation occurs in the QRS complex configuration in any of the ECG leads (see Figure 13–4B). These diagnoses may be difficult, however, when a single-surface ECG lead is recorded. As used in the literature, sustained VT is a tachycardia that lasts for 30 seconds or requires intervention for termination. The definition does not imply that a prolonged episode of VT lasting less than 30 seconds has any less clinical significance. Nonsustained VT is a term used to describe any three or more consecutive QRS complexes of ventricular origin with a rate of more than 100 bpm. When describing nonsustained VT, it is important to mention the number of complexes and rate in order to convey a clear picture of the event. In this chapter, VT is considered sustained unless specifically stated otherwise.
VT is most commonly associated with structural heart disease; 15–20% of VT episodes reported in various series is seen in patients without structural heart disease and is referred to as idiopathic VT. This distinction is important because the therapeutic approach to VT is different in patients with structural heart disease compared with patients with idiopathic VT in which case catheter ablation can be curative.
VENTRICULAR TACHYCARDIA ASSOCIATED WITH STRUCTURAL HEART DISEASE
1. Monomorphic Ventricular Tachycardia in Association with Chronic Coronary Artery Disease
This is the most common form encountered in clinical practice as well as the most extensively investigated in clinical and electrophysiology laboratories. The underlying substrate is usually an area of fibrosis that provides an anatomic obstacle around which the reentrant impulse can propagate. The extent and architecture of the scar may determine the propensity to VT in a given situation. For example, a homogeneous scar with no surviving conducting tissue is less likely to cause arrhythmias than is fibrosis interspersed with streaks of healthy myocardium. In some situations, acute myocardial ischemia or infarct can produce the conditions of slow conduction and block necessary for reentry. Although nonreentrant mechanisms (eg, abnormal automaticity, triggered activity) may cause VT in these settings, reentry is the most common mechanism.
As myocardial activation spreads from the reentrant circuit, the resultant QRS morphology is determined by the direction of the activation vector. Because of the myocardial origin of this type of VT, some characteristic features are expected: (1) The initial part of the QRS complex will generally be inscribed slowly because of muscle-to-muscle propagation. (2) The resultant QRS width is often markedly increased because of this intramyocardial conduction delay. (3) Because the impulse is not activating the ventricles via the bundle branches, the QRS pattern is not likely to be typical of either the RBBB or LBBB. (4) When there is no septal scar, VT originating in the left ventricle activates the ipsilateral ventricle, followed by the interventricular septum and then the right ventricle. This causes an atypical RBBB pattern. (5) VT that originates in the right ventricle shows a LBBB pattern. (6) When the tachycardia originates close to a septal scar, it may show an LBBB pattern even if the reentrant circuit resides in the left ventricle. (7) ECG leads can be used to localize the site of origin of VT from the left ventricle as follows: (a) Positive QRS complex in lead avR and negative in lead V4 suggests apical site of origin and vice versa for basal sites. (b) Positive QRS complexes in leads II, III, and avF suggest anterior site of origin, whereas negative QRS complex in these leads is seen in VT arising from inferior wall. (c) Positive QRS complex in leads I and avL is seen in VT arising from the septum, whereas a negative QRS in these leads suggests origin from the lateral wall. Figure 13–5 shows scar-related VT in a patient with previous history of MI. The VT has one reentrant circuit in the left ventricle with two different morphologies based on different exit sites.
After an initial documentation on the surface ECG, or when VT is suspected by virtue of underlying coronary artery disease and symptoms such as syncope, a further workup is often critical for characterization of VT. Ambulatory monitoring is of limited value because sustained VT is not commonly seen on a daily basis. In most situations, invasive electrophysiologic studies are indicated for these patients for both diagnostic and therapeutic purposes.
2. Monomorphic Ventricular Tachycardia in Association with Idiopathic Dilated Cardiomyopathy
Monomorphic VT associated with idiopathic dilated cardiomyopathy and valvular disease is indistinguishable from VT in chronic coronary artery disease in approximately 60% of patients. The underlying substrate is most probably fibrosis, providing both the anatomic obstacle and the pathway for reexcitation. Invariably, however, these patients have conduction slowing in the His-Purkinje system as a part of the diffuse myocardial disease process, and the baseline ECG shows evidence of nonspecific intraventricular conduction defect. The resultant tachycardia uses the bundle branches and the bundle of His for sustained reentry. Bundle branch reentry could manifest as either an LBBB or RBBB pattern (Figure 13–6). Because ventricular myocardial activation occurs via the bundle branches, a typical LBBB or RBBB configuration is generally noted. Although the prevalence of bundle branch reentry is particularly high in patients with idiopathic dilated cardiomyopathy, it does occur in dilated cardiomyopathy regardless of the underlying pathology.
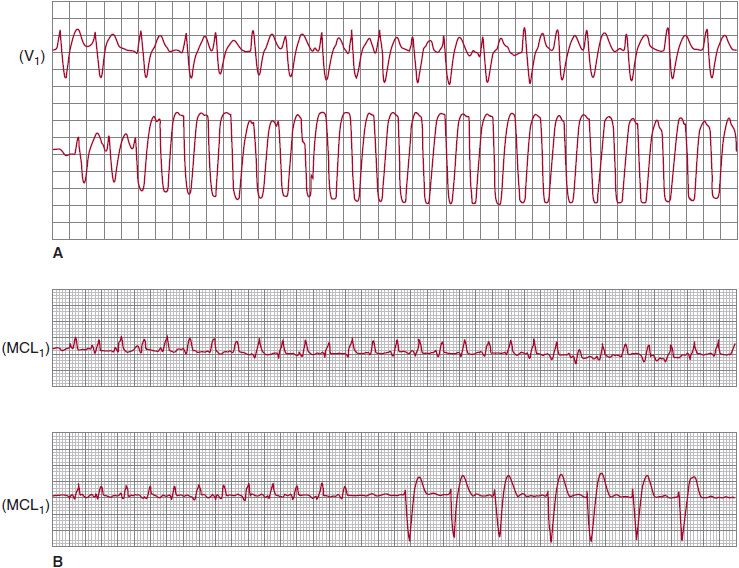
![]() Figure 13–6. Bundle branch reentrant ventricular tachycardia with typical left (A) and right (B) bundle branch morphologies, recorded at different times in a patient with idiopathic dilated cardiomyopathy. Note the underlying intraventricular conduction defect during baseline rhythm, before onset (A) and after termination (B). (Reproduced, with permission, from Tchou P, et al. Circulation 1988;78:246.)
Figure 13–6. Bundle branch reentrant ventricular tachycardia with typical left (A) and right (B) bundle branch morphologies, recorded at different times in a patient with idiopathic dilated cardiomyopathy. Note the underlying intraventricular conduction defect during baseline rhythm, before onset (A) and after termination (B). (Reproduced, with permission, from Tchou P, et al. Circulation 1988;78:246.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree