Approach to Cardiac Disease Diagnosis
Michael H. Crawford, MD
 General Considerations
General Considerations
The patient’s history is a critical feature in the evaluation of suspected or overt heart disease. It includes information about the present illness, past illnesses, and the patient’s family. From this information, a chronology of the patient’s disease process should be constructed. Determining what information in the history is useful requires a detailed knowledge of the pathophysiology of cardiac disease. The effort spent on listening to the patient is time well invested because the cause of cardiac disease is often discernible from the history.
A. Common Symptoms
1. Chest pain—Chest pain is one of the cardinal symptoms (Table 5–1) of ischemic heart disease, but it can also occur with other forms of heart disease. The five characteristics of ischemic chest pain, or angina pectoris, are as follows:
Table 5–1. Common Symptoms of Potential Cardiac Origin

• Anginal pain usually has a substernal location but may extend to the left or right chest, the shoulders, the neck, jaw, arms, epigastrium, and, occasionally, the upper back.
• The pain is deep, visceral, and intense; it makes the patient pay attention but is not excruciating. Many patients describe it as a pressure-like sensation or a tightness.
• The duration of the pain is minutes, not seconds.
• The pain tends to be precipitated by exercise or emotional stress.
• The pain is relieved by resting or taking sublingual nitroglycerin.
2. Dyspnea—A frequent complaint of patients with a variety of cardiac diseases, dyspnea is ordinarily one of four types. The most common is exertional dyspnea, which usually means that the underlying condition is mild because it requires the increased demand of exertion to precipitate symptoms. The next most common is paroxysmal nocturnal dyspnea, characterized by the patient awakening after being asleep or recumbent for an hour or more. This symptom is caused by the redistribution of body fluids from the lower extremities into the vascular space and back to the heart, resulting in volume overload; it suggests a more severe condition. Third is orthopnea, a dyspnea that occurs immediately on assuming the recumbent position. The mild increase in venous return (caused by lying down) before any fluid is mobilized from interstitial spaces in the lower extremities is responsible for the symptom, which suggests even more severe disease. Finally, dyspnea at rest suggests severe cardiac disease.
Dyspnea is not specific for heart disease, however. Exertional dyspnea, for example, can be due to pulmonary disease, anemia, or deconditioning. Orthopnea is a frequent complaint in patients with chronic obstructive pulmonary disease and postnasal drip. A history of “two-pillow orthopnea” is of little value unless the reason for the use of two pillows is discerned. Resting dyspnea is also a sign of pulmonary disease. Paroxysmal nocturnal dyspnea is perhaps the most specific for cardiac disease because few other conditions cause this symptom.
3. Syncope and presyncope—Lightheadedness, dizziness, presyncope, and syncope are important indications of a reduction in cerebral blood flow. These symptoms are nonspecific and can be due to primary central nervous system disease, metabolic conditions, dehydration, or inner-ear problems. Because bradyarrhythmias and tachyarrhythmias are important cardiac causes, a history of palpitations preceding the event is significant.
4. Transient central nervous system deficits—Deficits such as transient ischemic attacks (TIAs) suggest emboli from the heart or great vessels or, rarely, from the venous circulation through an intracardiac shunt. A TIA should prompt the search for cardiovascular disease. Any sudden loss of blood flow to a limb also suggests a cardioembolic event.
5. Fluid retention—These symptoms are not specific for heart disease but may be due to reduced cardiac function. Typical symptoms are peripheral edema, bloating, weight gain, and abdominal pain from an enlarged liver or spleen. Decreased appetite, diarrhea, jaundice, and nausea and vomiting can also occur from gut and hepatic dysfunction due to fluid engorgement.
6. Palpitation—Normal resting cardiac activity usually cannot be appreciated by the individual. Awareness of heart activity is often referred to by patients as palpitation. Among patients, there is no standard definition for the type of sensation represented by palpitation, so the physician must explore the sensation further with the patient. It is frequently useful to have the patient tap the perceived heartbeat out by hand. Commonly, unusually forceful heart activity at a normal rate (60–100 bpm) is perceived as palpitation. More forceful contractions are usually the result of endogenous catecholamine excretion that does not elevate the heart rate out of the normal range. A common cause of this phenomenon is anxiety. Another common sensation is that of the heart stopping transiently or of the occurrence of isolated forceful beats or both. This sensation is usually caused by premature ventricular contractions, and the patient either feels the compensatory pause or the resultant more forceful subsequent beat or both. Occasionally, the individual feels the ectopic beat and refers to this phenomenon as “skipped” beats. The least common sensation reported by individuals, but the one most linked to the term “palpitation” is rapid heart rate that may be regular or irregular and is usually supraventricular in origin.
7. Cough—Although cough is usually associated with pulmonary disease processes, cardiac conditions that lead to pulmonary abnormalities may be the root cause of the cough. A cardiac cough is usually dry or nonproductive. Pulmonary fluid engorgement from conditions such as heart failure may present as cough. Pulmonary hypertension from any cause can result in cough. Finally, angiotensin-converting enzyme inhibitors, which are frequently used in cardiac conditions, can cause cough.
B. History
1. The present illness—This is a chronology of the events leading up to the patient’s current complaints. Usually physicians start with the chief complaint and explore the patient’s symptoms. It is especially important to determine the frequency, intensity, severity, and duration of all symptoms; their precipitating causes; what relieves them; and what aggravates them. Although information about previous related diseases and opinions from other physicians are often valuable, it is essential to explore the basis of any prior diagnosis and ask the patient about objective testing and the results of such testing. A history of prior treatment is often revealing because medications or surgery may indicate the nature of the original problem. A list should be made of all the patient’s current medications, detailing the dosages, the frequency of administration, whether they are helping the patient, and any side effects.
2. Antecedent conditions—Several systemic diseases may have cardiac involvement. It is therefore useful to search for a history of rheumatic fever, which may manifest as Sydenham chorea, joint pain and swelling, or merely frequent sore throats. Other important diseases that affect the heart include metastatic cancer, thyroid disorders, diabetes mellitus, and inflammatory diseases such as rheumatoid arthritis and systemic lupus erythematosus. Certain events during childhood are suggestive of congenital or acquired heart disease; these include a history of cyanosis, reduced exercise tolerance, or long periods of restricted activities or school absence. Exposure to toxins, infectious agents, and other noxious substances may also be relevant.
3. Atherosclerotic risk factors—Atherosclerotic cardiovascular disease is the most common form of heart disease in industrialized nations. The presenting symptoms of this ubiquitous disorder may be unimpressive and minimal, or as impressive as sudden death. It is therefore important to determine from the history whether any risk factors for this disease are present. The most important are a family history of atherosclerotic disease, especially at a young age; diabetes mellitus; lipid disorders such as a high cholesterol level; hypertension; and smoking. Less important factors include a lack of exercise, high stress levels, the type A personality, and truncal obesity.
4. Family history—A family history is important for determining the risk for not only atherosclerotic cardiovascular disease but also for many other cardiac diseases. Congenital heart disease, for example, is more common in the off-spring of parents with this condition, and a history of the disorder in the antecedent family or siblings is significant. Other genetic diseases, such as neuromuscular disorders or connective tissue disorders (eg, Marfan syndrome), can affect the heart. Acquired diseases, such as rheumatic valve disease, can cluster in families because of the spread of the streptococcal infection among family members. The lack of a history of hypertension in the family might prompt a more intensive search for a secondary cause. A history of atherosclerotic disease sequelae, such as limb loss, strokes, and heart attacks, may provide a clue to the aggressiveness of an atherosclerotic tendency in a particular family group.
 Physical Findings
Physical Findings
A. Physical Examination
The physical examination is less important than the history in patients with ischemic heart disease, but it is of critical value in patients with congenital and valvular heart disease. In the latter two categories, the physician can often make specific anatomic and etiologic diagnoses based on the physical examination. Certain abnormal murmurs and heart sounds are specific for structural abnormalities of the heart. The physical examination is also important for confirming the diagnosis and establishing the severity of heart failure, and it is the only way to diagnose systemic hypertension because this diagnosis is based on elevated blood pressure recordings.
1. Blood pressure—Proper measurement of the systemic arterial pressure by cuff sphygmomanometry is one of the keystones of the cardiovascular physical examination. It is recommended that the brachial artery be palpated and the diaphragm of the stethoscope be placed over it, rather than merely sticking the stethoscope in the antecubital fossa. Current methodologic standards dictate that the onset and disappearance of the Korotkoff sounds define the systolic and diastolic pressures, respectively. Although this is the best approach in most cases, there are exceptions. For example, in patients in whom the diastolic pressure drops to near zero, the point of muffling of the sounds is usually recorded as the diastolic pressure. Because the diagnosis of systemic hypertension involves repeated measures under the same conditions, the operator should record the arm used and the position of the patient to allow reproducible measurements to be made on serial visits.
Orthostatic changes in blood pressure are a very important physical finding, especially in patients complaining of transient central nervous system symptoms, weakness, or unstable gait. The technique involves having the patient assume the upright position for at least 90 seconds before taking the pressure to be sure that the maximum orthostatic effect is measured. Although measuring the pressure in other extremities may be of value in certain vascular diseases, it provides little information in a routine examination beyond palpating pulses in all the extremities. Keep in mind, in general, that the pulse pressure (the difference between systolic and diastolic blood pressures) is a crude measure of left ventricular stroke volume. A widened pulse pressure suggests that the stroke volume is large; a narrowed pressure, that the stroke volume is small.
2. Peripheral pulses—When examining the peripheral pulses, the physician is really conducting three examinations. The first is an examination of the cardiac rate and rhythm, the second is an assessment of the characteristics of the pulse as a reflection of cardiac activity, and the third is an assessment of the adequacy of the arterial conduit being examined. The pulse rate and rhythm are usually determined in a convenient peripheral artery, such as the radial. If a pulse is irregular, it is better to auscultate the heart; some cardiac contractions during rhythm disturbances do not generate a stroke volume sufficient to cause a palpable peripheral pulse. In many ways, the heart rate reflects the health of the circulatory system. A rapid pulse suggests increased catecholamine levels, which may be due to cardiac disease, such as heart failure; a slow pulse represents an excess of vagal tone, which may be due to disease or athletic training.
To assess the characteristics of the cardiac contraction through the pulse, it is usually best to select an artery close to the heart, such as the carotid. Bounding high-amplitude carotid pulses suggest an increase in stroke volume and should be accompanied by a wide pulse pressure on the blood pressure measurement. A weak carotid pulse suggests a reduced stroke volume. Usually the strength of the pulse is graded on a scale of 1 to 4, where 2 is a normal pulse amplitude, 3 or 4 is a hyperdynamic pulse, and 1 is a weak pulse. A low-amplitude, slow-rising pulse, which may be associated with a palpable vibration (thrill), suggests aortic stenosis. A bifid pulse (beating twice in systole) can be a sign of hypertrophic obstructive cardiomyopathy, severe aortic regurgitation, or the combination of moderately severe aortic stenosis and regurgitation. A dicrotic pulse (an exaggerated, early, diastolic wave) is found in severe heart failure. Pulsus alternans (alternate strong and weak pulses) is also a sign of severe heart failure. When evaluating the adequacy of the arterial conduits, all palpable pulses can be assessed and graded on a scale of 0 to 4, where 4 is a fully normal conduit, and anything below that is reduced, including 0—which indicates an absent pulse. The major pulses routinely palpated on physical examination are the radial, brachial, carotid, femoral, dorsalis pedis, and posterior tibial. In special situations, the abdominal aorta and the ulnar, subclavian, popliteal, axillary, temporal, and intercostal arteries are palpated. In assessing the abdominal aorta, it is important to make note of the width of the aorta because an increase suggests an abdominal aortic aneurysm. It is particularly important to palpate the abdominal aorta in older individuals because abdominal aortic aneurysms are more prevalent in those older than 70. An audible bruit is a clue to significantly obstructed large arteries. During a routine examination, bruits are sought with the stethoscope head placed over the carotids, abdominal aorta, and femorals at the groin. Other arteries may be auscultated under special circumstances, such as suspected renal artery stenosis (flank bruit).
3. Jugular venous pulse—Assessment of the jugular venous pulse can provide information about the central venous pressure and right-heart function. Examination of the right internal jugular vein is ideal for assessing central venous pressure because it is attached directly to the superior vena cava without intervening valves. The patient is positioned into the semiupright posture that permits visualization of the top of the right internal jugular venous blood column. The height of this column of blood, vertically from the sternal angle, is added to 5 cm of blood (the presumed distance to the center of the right atrium from the sternal angle) to obtain an estimate of central venous pressure in centimeters of blood. This can be converted to millimeters of mercury (mm Hg) with the formula:
mm Hg = cm blood × 0.736.
Examining the characteristics of the right internal jugular pulse is valuable for assessing right-heart function and rhythm disturbances. The normal jugular venous pulse has two distinct waves: a and v; the former coincides with atrial contraction and the latter with late ventricular systole. An absent a wave and an irregular pulse suggest atrial fibrillation. A large and early v wave suggests tricuspid regurgitation. The dips after the a and v waves are the x and y descents; the former coincide with atrial relaxation and the latter with early ventricular filling. In tricuspid stenosis, the y descent is prolonged. Other applications of the jugular pulse examination are discussed in the chapters dealing with specific disorders.
4. Lungs—Evaluation of the lungs is an important part of the physical examination: Diseases of the lung can affect the heart, just as diseases of the heart can affect the lungs. The major finding of importance is rales at the pulmonary bases, indicating alveolar fluid collection. Although this is a significant finding in patients with congestive heart failure, it is not always possible to distinguish rales caused by heart failure from those caused by pulmonary disease. The presence of pleural fluid, although useful in the diagnosis of heart failure, can be due to other causes. Heart failure most commonly causes a right pleural effusion; it can cause effusions on both sides but is least likely to cause isolated left pleural effusion. The specific constellation of dullness at the left base with bronchial breath sounds suggests an increase in heart size from pericardial effusion (Ewart sign) or another cause of cardiac enlargement; it is thought to be due to compression by the heart of a left lower lobe bronchus.
When right-heart failure develops or venous return is restricted from entering the heart, venous pressure in the abdomen increases, leading to hepatosplenomegaly and eventually ascites. None of these physical findings is specific for heart disease; they do, however, help establish the diagnosis. Heart failure also leads to generalized fluid retention, usually manifested as lower extremity edema or, in severe heart failure, anasarca.
5. Cardiac auscultation—Heart sounds are caused by the acceleration and deceleration of blood and the subsequent vibration of the cardiac structures during the phases of the cardiac cycle. To hear cardiac sounds, use a stethoscope with a bell and a tight diaphragm. Low-frequency sounds are associated with ventricular filling and are heard best with the bell. Medium-frequency sounds are associated with valve opening and closing; they are heard best with the diaphragm. Cardiac murmurs are due to turbulent blood flow, are usually high-to-medium frequency, and are heard best with the diaphragm. Low-frequency atrioventricular valve inflow murmurs, such as that produced by mitral stenosis, are best heard with the bell, however. Auscultation should take place in areas that correspond to the location of the heart and great vessels. Such placement will, of course, need to be modified for patients with unusual body habitus or an unusual cardiac position. When no cardiac sounds can be heard over the precordium, they can often be heard in either the subxiphoid area or the right supraclavicular area.
Auscultation in various positions is recommended because low-frequency filling sounds are best heard with the patient in the left lateral decubitus position, and high-frequency murmurs, such as that of aortic regurgitation, are best heard with the patient sitting.
A. HEART SOUNDS—The first heart sound is coincident with mitral and tricuspid valve closure and has two components in up to 40% of normal individuals. There is little change in the intensity of this sound with respiration or position. The major determinant of the intensity of the first heart sound is the electrocardiographic (ECG) PR interval, which determines the time delay between atrial and ventricular contraction and thus the position of the mitral valve when ventricular systole begins. With a short PR interval, the mitral valve is widely open when systole begins, and its closure increases the intensity of the first sound, as compared to a long PR-interval beat when the valve partially closes prior to the onset of ventricular systole. Certain disease states, such as mitral stenosis, also can increase the intensity of the first sound.
The second heart sound is coincident with closure of the aortic and pulmonic valves. Normally, this sound is single in expiration and split during inspiration, permitting the aortic and pulmonic components to be distinguished. The inspira-tory split is due to a delay in the occurrence of the pulmonic component because of a decrease in pulmonary vascular resistance, which prolongs pulmonary flow beyond the end of right ventricular systole. Variations in this normal splitting of the second heart sound are useful in determining certain disease states. For example, in atrial septal defect, the second sound is usually split throughout the respiratory cycle because of the constant increase in pulmonary flow. In patients with left bundle branch block, a delay occurs in the aortic component of the second heart sound, which results in reversed respiratory splitting; single with inspiration, split with expiration.
A third heart sound occurs during early rapid filling of the left ventricle; it can be produced by any condition that causes left ventricular volume overload or dilatation. It can therefore be heard in such disparate conditions as congestive heart failure and normal pregnancy. A fourth heart sound is due to a vigorous atrial contraction into a stiffened left ventricle and can be heard in left ventricular hypertrophy of any cause or in diseases that reduce compliance of the left ventricle, such as myocardial infarction.
Although third and fourth heart sounds can occasionally occur in normal individuals, all other extra sounds are signs of cardiac disease. Early ejection sounds are due to abnormalities of the semilunar valves, from restriction of their motion, thickening, or both (eg, a bicuspid aortic valve, pulmonic or aortic stenosis). A midsystolic click is often due to mitral valve prolapse and is caused by sudden tensing in midsystole of the redundant prolapsing segment of the mitral leaflet. The opening of a thickened atrioventricular valve leaflet, as in mitral stenosis, will cause a loud opening sound (snap) in early diastole. A lower frequency (more of a knock) sound at the time of rapid filling may be an indication of constrictive pericarditis. These early diastolic sounds must be distinguished from a third heart sound.
B. MURMURS—Systolic murmurs are very common and do not always imply cardiac disease. They are usually rated on a scale of 1 to 6, where grade 1 is barely audible, grade 4 is associated with palpable vibrations (thrill), grade 5 can be heard with the edge of the stethoscope, and grade 6 can be heard without a stethoscope. Most murmurs fall in the 1–3 range, and murmurs in the 4–6 range are almost always due to pathologic conditions; severe disease can exist with grades 1–3 or no cardiac murmurs, however. The most common systolic murmur is the crescendo/decrescendo murmur that increases in intensity as blood flows early in systole and diminishes in intensity through the second half of systole. This murmur can be due to vigorous flow in a normal heart or to obstructions in flow, as occurs with aortic stenosis, pulmonic stenosis, or hypertrophic cardiomyopathy. The so-called innocent flow murmurs are usually grades 1–2 and occur very early in systole; they may have a vibratory quality and are usually less apparent when the patient is in the sitting position (when venous return is less). If an ejection sound is heard, there is usually some abnormality of the semilunar valves. Although louder murmurs may be due to pathologic cardiac conditions, this is not always so. Distinguishing benign from pathologic systolic flow murmurs is one of the major challenges of clinical cardiology. Benign flow murmurs can be heard in 80% of children; the incidence declines with age, but may be prominent during pregnancy or in adults who are thin or physically well trained. The murmur is usually benign in a patient with a soft flow murmur that diminishes in intensity in the sitting position and neither a history of cardiovascular disease nor other cardiac findings.
The holosystolic, or pansystolic, murmur is almost always associated with cardiac pathology. The most common cause of this murmur is atrioventricular valve regurgitation, but it can also be observed in conditions such as ventricular septal defect, in which an abnormal communication exists between two chambers of markedly different systolic pressures. Although it is relatively easy to determine that these murmurs represent an abnormality, it is more of a challenge to determine their origins. Keep in mind that such conditions as mitral regurgitation, which usually produce holosystolic murmurs, may produce crescendo/decrescendo murmurs, adding to the difficulty in differentiating benign from pathologic systolic flow murmurs.
Diastolic murmurs are always abnormal and are usually graded on a 1 to 4 scale. The most frequently heard diastolic murmur is the high-frequency decrescendo early diastolic murmur of aortic regurgitation. This is usually heard best at the upper left sternal border or in the aortic area (upper right sternal border) and may radiate to the lower left sternal border and the apex. This murmur is usually very high frequency and may be difficult to hear. Although the murmur of pulmonic regurgitation may sound like that of aortic regurgitation when pulmonary artery pressures are high, if structural disease of the valve is present with normal pulmonary pressures, the murmur usually has a midrange frequency and begins with a slight delay after the pulmonic second heart sound. Pulmonic regurgitation is usually best heard in the pulmonic area (left second intercostal space parasternally). Mitral stenosis produces a low-frequency rumbling diastolic murmur that is decrescendo in early diastole, but may become crescendo up to the first heart sound with moderately severe mitral stenosis and sinus rhythm. The murmur is best heard at the apex in the left lateral decubitus position with the bell of the stethoscope. Similar findings are heard in tricuspid stenosis, but the murmur is loudest at the lower left sternal border.
A continuous murmur implies a connection between a high- and a low-pressure chamber throughout the cardiac cycle, such as occurs with a fistula between the aorta and the pulmonary artery. If the connection is a patent ductus arteriosus, the murmur is heard best under the left clavicle; it has a machine-like quality. Continuous murmurs must be distinguished from the combination of systolic and diastolic murmurs in patients with combined lesions (eg, aortic stenosis and regurgitation).
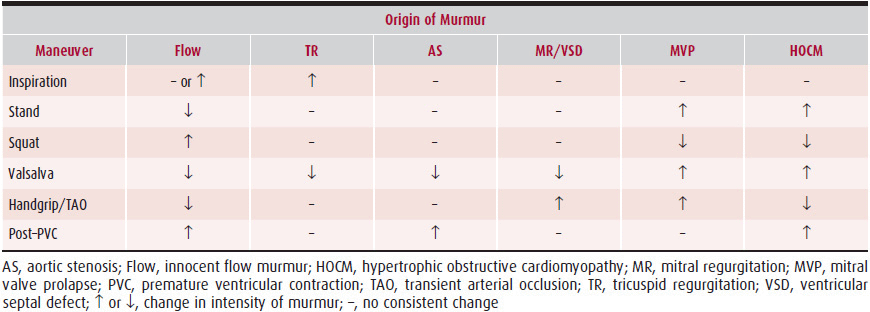
Traditionally, the origin of heart murmurs was based on five factors: (1) their timing in the cardiac cycle, (2) where on the chest they were heard, (3) their characteristics, (4) their intensity, and (5) their duration. Unfortunately, this traditional classification system is unreliable in predicting the underlying pathology. A more accurate method, dynamic auscultation, changes the intensity, duration, and characteristics of the murmur by bedside maneuvers that alter hemodynamics.
The simplest of these maneuvers is observation of any changes in murmur intensity with normal respiration because all right-sided cardiac murmurs should increase in intensity with normal inspiration. Although some exceptions exist, the method is very reliable for detecting such murmurs. Inspiration is associated with reductions in intrathoracic pressure that increase venous return from the abdomen and the head, leading to an increased flow through the right heart chambers. The consequent increase in pressure increases the intensity of right-sided murmurs. These changes are best observed in the sitting position, where venous return is smallest, and changes in intrathoracic pressure can produce their greatest effect on venous return. In a patient in the supine position, when venous return is near maximum, there may be little change observed with respiration. The ejection sound caused by pulmonic stenosis does not routinely increase in intensity with inspiration. The increased blood in the right heart accentuates atrial contraction, which increases late diastolic pressure in the right ventricle, partially opening the stenotic pulmonary valve and thus diminishing the opening sound of this valve with the subsequent systole.
Changes in position are an important part of normal auscultation; they can also be of great value in determining the origin of cardiac murmurs (Table 5–2). Murmurs dependent on venous return, such as innocent flow murmurs, are softer or absent in upright positions; others, such as the murmur associated with hypertrophic obstructive cardiomyopathy, are accentuated by reduced left ventricular volume associated with the upright position. In physically capable individuals, a rapid squat from the standing position is often diagnostically valuable because it suddenly increases venous return and left ventricular volume and accentuates flow murmurs but diminishes the murmur of hypertrophic obstructive cardiomyopathy. The stand-squat maneuver is also useful for altering the timing of the midsystolic click caused by mitral valve prolapse during systole. When the ventricle is small during standing, the prolapse occurs earlier in systole, moving the midsystolic click to early systole. During squatting, the ventricle dilates and the prolapse is delayed in systole, resulting in a late midsystolic click.
Table 5–2. Differentiation of Systolic Murmurs Based on Changes in Their Intensity from Physiologic Maneuvers
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


