Heart Failure with Preserved Ejection Fraction
Sanjiv J. Shah, MD
 ESSENTIALS OF DIAGNOSIS
ESSENTIALS OF DIAGNOSIS
![]() Symptoms and signs of heart failure with preserved left ventricular ejection fraction (LVEF > 50%).
Symptoms and signs of heart failure with preserved left ventricular ejection fraction (LVEF > 50%).
![]() Presence of an underlying cause of heart failure with preserved ejection fraction (eg, comorbidities such as hypertension, coronary artery disease, diabetes, chronic kidney disease; or underlying valvular heart disease, restrictive cardiomyopathy, or specific myocardial diseases such as amyloidosis).
Presence of an underlying cause of heart failure with preserved ejection fraction (eg, comorbidities such as hypertension, coronary artery disease, diabetes, chronic kidney disease; or underlying valvular heart disease, restrictive cardiomyopathy, or specific myocardial diseases such as amyloidosis).
![]() Objective evidence of elevated left ventricular filling pressure (at rest or with exercise) on echocardiography or cardiac catheterization.
Objective evidence of elevated left ventricular filling pressure (at rest or with exercise) on echocardiography or cardiac catheterization.
 General Considerations
General Considerations
Heart failure with preserved ejection fraction (HFpEF) is an increasingly common, debilitating syndrome of the elderly, and one that carries a high rate of morbidity and mortality. HFpEF accounts for > 50% of all hospitalizations for heart failure, and while a recent patient-level meta-analysis found that patients with heart failure and reduced ejection fraction (HFrEF) have a worse prognosis compared to HFpEF, two earlier large epidemiologic studies found that patients with HFpEF have a mortality rate that is nearly identical to HFrEF. Regardless of underlying ejection fraction, survival for all heart failure (HF) patients is poor, especially after HF hospitalization.
HFpEF is the preferred term for patients with a normal ejection fraction who have the syndrome of HF, because HFpEF highlights the fact that HF is a syndrome and not a distinct clinical or pathophysiologic entity. Many investigators and experts have used the term “diastolic heart failure” for HFpEF in the past. However, this term is not ideal for two main reasons. First, there is ample evidence that patients with HFpEF have abnormalities in systolic function (as defined by tissue Doppler imaging) despite a normal ejection fraction, and many patients with HFrEF have abnormal diastolic function. Second, in the clinical setting, patients with HF are currently classified into two categories: low ejection fraction (< 50%) and preserved ejection fraction (> 50%). By calling HFpEF “diastolic HF,” clinicians may not consider the entire differential diagnosis of HFpEF (of which pure diastolic dysfunction is only one cause). HFpEF has also previously been called “HF with preserved systolic function” or “HF with normal systolic function.” As stated earlier, it is now clear that many patients with HFpEF have abnormalities in systolic function; therefore, HFpEF is a better term.
Finally, HFpEF has the advantage of being an easy mnemonic for patients to remember. HFpEF sounds like “HUFF-PUFF,” which helps patients understand this syndrome, in which dyspnea and fatigue are two of the most common symptoms.
Borlaug BA, et al. Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–9. [PMID: 21138935]
Maeder MT, et al. Heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2009;53(11):905–18. [PMID: 19281919]
Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. 2012;33(14):1750–7. [PMID: 21821849]
 Pathophysiology
Pathophysiology
Since HFpEF is heterogeneous, there is no single mechanism that can explain the pathophysiology of the HFpEF syndrome. In some patients with HFpEF, such as those who have the signs and symptoms of HF due to severe valvular disease or pericardial disease (ie, constrictive pericarditis), pathophysiology is relatively straightforward and well-defined. However, in most patients with HFpEF, pathophysiologic abnormalities cannot be ascribed to a single well-defined mechanism. Instead, these patients typically have one or more of the following underlying pathophysiologic processes: (1) diastolic dysfunction due to impaired left ventricular (LV) relaxation, increased LV diastolic stiffness, or both; (2) LV enlargement with increased intravascular volume, which may be due to extracardiac factors such as renal insufficiency; (3) abnormal ventricular-arterial coupling with increased ventricular systolic stiffness and increased arterial stiffness; (4) right HF due to pulmonary venous hypertension with or without superimposed pulmonary arterial hypertension; and (5) chronotropic incompetence. In addition, LV hypertrophy and coronary artery disease are especially important in the pathophysiology of patients with HFpEF.
A. Diastolic Dysfunction
Diastolic dysfunction occurs when the ventricle loses its normal ability to suction blood from the left atrium. When the ventricle relaxes abnormally, filling is delayed and left atrial emptying is incomplete. An abnormally stiff ventricle worsens the problem by also impeding left atrial emptying. The end result is abnormally high left atrial and LV diastolic pressures. The LV loses its suction and instead of “pulling” blood from the left atrium and pulmonary veins, it now relies heavily on left atrial contraction so that the LV can fill and distend appropriately and recoil in systole. This is one reason why atrial fibrillation is tolerated so poorly in patients with advanced LV diastolic dysfunction with resultant elevation of left atrial pressure, pulmonary vascular congestion, and poor cardiac output.
In patients with HFpEF who have substantial diastolic dysfunction as a cause of their symptoms, the end-diastolic pressure-volume relationship is shifted up and to the left (Figure 27–1). In these patients, even small increases in central blood volume or vascular (arterial or venous) tone can result in significant increases in left atrial volume and pulmonary venous pressures. Patients with an upward and leftward shift in the LV end-diastolic pressure–volume relationship tend to have a high relative wall thickness (high LV mass/volume ratio); increased fibrosis and scar of the LV myocardium due to ischemia, infarction, infiltrative disease, or radiation; and impaired active relaxation of the myocardium (due to abnormal myocyte calcium homeostasis).
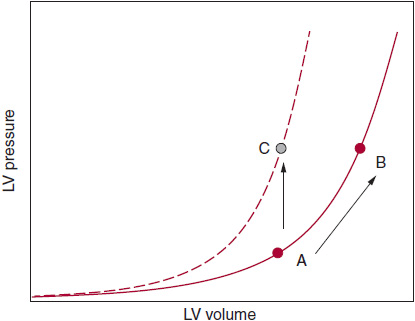
![]() Figure 27–1. In patients who have a noncompliant left ventricle (LV), the end-diastolic pressure-volume relationship (EDPVR) is displaced upward and to the left; therefore, there is diminished capacity to fill at low LV filling pressures. A = normal LV compliance; B = stiff, noncompliant LV with upward and leftward-shifted EDPVR; C = normal LV compliance with volume overload (note that the EDPVR curve does not shift but LV filling pressure is increased). It is difficult to distinguish scenario A from scenario C on echocardiography alone (ie, both scenarios could result in grade II diastolic dysfunction on echocardiography). Thus, “diastolic dysfunction” determined by echocardiography can help diagnose elevated LV filling pressures (and impaired LV relaxation), but it is harder to diagnose reduced LV compliance by echocardiography alone.
Figure 27–1. In patients who have a noncompliant left ventricle (LV), the end-diastolic pressure-volume relationship (EDPVR) is displaced upward and to the left; therefore, there is diminished capacity to fill at low LV filling pressures. A = normal LV compliance; B = stiff, noncompliant LV with upward and leftward-shifted EDPVR; C = normal LV compliance with volume overload (note that the EDPVR curve does not shift but LV filling pressure is increased). It is difficult to distinguish scenario A from scenario C on echocardiography alone (ie, both scenarios could result in grade II diastolic dysfunction on echocardiography). Thus, “diastolic dysfunction” determined by echocardiography can help diagnose elevated LV filling pressures (and impaired LV relaxation), but it is harder to diagnose reduced LV compliance by echocardiography alone.
B. Left Ventricular Enlargement and Increased Intravascular Volume
LV enlargement is a key predictor of HF, regardless of ejection fraction. However, patients with isolated diastolic HF are often thought to have small LV volumes. This apparent discrepancy can be explained by the underlying cause of HFpEF and diastolic HF. It is likely that patients with significant coronary disease or myocardial ischemia (even in the absence of epicardial coronary disease) suffer from increased LV enlargement. In addition, many patients with LV enlargement have increased intravascular volume due to comorbidities such as chronic kidney disease, anemia, and obesity. Thus, LV enlargement and increased intravascular volume cause symptoms of HFpEF by a pathophysiologic mechanism that is distinct from pure LV diastolic dysfunction.
C. Abnormal Ventricular-Arterial Coupling
Ventricular-arterial coupling describes the interaction between ventricular stiffness and central arterial stiffness. In healthy patients, young and old, arterial and ventricular elastance (stiffness) are matched in order to maintain optimal cardiac efficiency. However, with increasing age, ventricular stiffness is elevated and results in decreased contractile reserve, thereby rendering elderly patients susceptible to HF, blood pressure lability, and decreased exercise tolerance. Some patients with HFpEF appear to be particularly susceptible to abnormal ventricular-arterial coupling. These patients have the age-related increases in ventricular stiffness described earlier, but instead of matched ventricular and arterial stiffness, ventricular stiffness rises out of proportion of arterial stiffness, which results in poor cardiac efficiency. These patients tend to have high pulse pressure, and they tend to be most sensitive to diuretics whereby small changes in blood volume result in large changes in blood pressure (either significantly hypertensive or hypotensive).
D. Right Heart Failure
Elevated pulmonary artery systolic pressure (PASP) on echocardiography is present in > 75% of patients with HFpEF and is a marker of worse outcomes in HFpEF. In addition, elevated PASP is the echocardiographic finding that has the best test characteristics for differentiating patients with HFpEF from those with systemic hypertension without HF. It is not surprising that elevated PASP and pulmonary hypertension are common in HFpEF since elevated left atrial pressure (at rest and/or with exercise) is present almost universally in these patients. Elevated left atrial pressure results in increased pulmonary venous pressures, thereby causing pulmonary hypertension. Significant systemic hypertension, which also occurs commonly in HFpEF, also contributes to the high PASP. Thus, pulmonary venous hypertension, defined invasively as mean pulmonary artery pressure (mPAP) > 25 mm Hg with a pulmonary capillary wedge pressure (PCWP) > 15 mm Hg, is common in HFpEF and contributes to right ventricular hypertrophy, dysfunction, and eventual right HF. Some patients (most likely < 5–10% of HFpEF) develop superimposed pulmonary arterial hypertension (on top of pulmonary venous hypertension) either as an extension of their severe pulmonary venous hypertension or due to other risk factors such as chronic thromboembolic disease, chronic hypoxemic lung disease, or obstructive sleep apnea. The best invasive marker for differentiating pulmonary venous hypertension from superimposed pulmonary arterial hypertension is not the transpulmonary gradient (mPAP-PCWP) or pulmonary vascular resistance; rather, it is the pulmonary vascular gradient (pulmonary artery diastolic pressure [PADP]-PCWP). If the PADP-PCWP gradient is > 5–10 mm Hg, pulmonary arterial hypertension is present; if the PADP and PCWP are equalized (ie, PADPPCWP < 5 mm Hg), only pulmonary venous hypertension is present, and there is no significant superimposed pulmonary arterial hypertension.
E. Chronotropic incompetence
Up to 20–30% of patients with HFpEF have evidence of chronotropic incompetence. The inability to increase heart rate with exercise causes exercise intolerance. Patients with advanced HFpEF in particular, such as those with restrictive cardiomyopathy, severe LV hypertrophy, or severe coronary artery disease, have a very stiff LV and are unable to augment stroke volume with exercise. Thus, these patients rely upon increasing heart rate to augment cardiac output with exercise. If chronotropic incompetence is present, exercise capacity will be very limited in these types of patients.
F. Left Ventricular Hypertrophy
LV hypertrophy contributes substantially to the pathophysiology of HFpEF and is an important risk factor. LV hypertrophy limits coronary flow reserve, increases LV diastolic stiffness, and impairs LV relaxation. Patients with LV hypertrophy suffer from an inability to adequately utilize the Frank-Starling mechanism. Therefore, inadequate preload and chronotropic incompetence can lead to decreased cardiac output, with resultant lightheadedness, dizziness, and exercise intolerance. Finally, because of increased LV wall thickness, the subendocardium is especially vulnerable to ischemia in patients with and without epicardial coronary disease due to decreased coronary blood flow during exercise. Subendocardial ischemia can cause both systolic and diastolic dysfunction in these patients and further exacerbate HFpEF.
G. Coronary Artery Disease
Approximately 50% of patients with HFpEF have concomitant coronary artery disease. In these patients, coronary disease is often severe, involving multiple epicardial coronary arteries. Patients with prior myocardial infarction, ongoing ischemia, or stable chronic coronary disease can all present with HFpEF. Myocardial ischemia causes calcium sequestration in diastole, which results in impaired LV relaxation and increased LV filling pressures. In areas of prior infarction or ongoing ischemia, regional systolic dysfunction and dyssynchrony can further exacerbate abnormal loading conditions and create a mixture of systolic and diastolic dysfunction. Furthermore, patients with chronic coronary artery disease often have LV remodeling with resultant ventricular enlargement, a known risk factor for increased mortality and HF, regardless of ejection fraction. Preservation of ejection fraction can occur in some patients with prior infarction due to hypertrophy and hyperdynamic function of noninfarcted areas.
Patients with coronary disease suffer from a vicious cycle of abnormalities that contribute to HFpEF. As noted earlier, ischemia can cause impaired LV relaxation and increased LV filling pressures. Impaired LV relaxation in turn can also adversely affect coronary blood flow and coronary flow reserve, which exacerbates ischemia. Increased LV filling pressures results in extravascular compression of the small intramyocardial coronary vessels, which can cause subendocardial ischemia. Increased LV end-diastolic pressure can also result in poor epicardial coronary blood flow. Thus, ischemia begets worsening LV diastolic function, which begets more ischemia.
Borlaug BA, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47. [PMID: 17088459]
Guazzi M, et al. Pulmonary hypertension due to left heart disease. Circulation. 2012;126(8):975–90. [PMID: 22908015]
Kliger C, et al. A clinical algorithm to differentiate heart failure with a normal ejection fraction by pathophysiologic mechanism. Am J Geriatr Cardiol. 2006;15(1):50–7. [PMID: 16415647]
Lam CS, et al. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119–26. [PMID: 19324256]
Maurer MS, et al. Left heart failure with a normal ejection fraction: identification of different pathophysiologic mechanisms. J Card Fail. 2005;11(3):177–87. [PMID: 15812744]
Shah SJ. Evolving approaches to the management of heart failure with preserved ejection fraction in patients with coronary artery disease. Curr Treat Options Cardiovasc Med. 2010;12(1):58–75. [PMID: 20842482]
Zile MR, et al. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–9. [PMID: 15128895]
 Clinical Findings
Clinical Findings
The first step in caring for a patient with HFpEF is to ensure the correct diagnosis (see later section on Differential Diagnosis). Several criteria for the diagnosis of HFpEF exist. All require signs and symptoms of HF and objective evidence of preserved LV ejection fraction (≥ 50%).
A. Risk Factors
1. Age—Patients with HFpEF are almost universally elderly, and aging has several effects on cardiovascular structure and function that are pertinent to HFpEF patients. Aging reduces the diastolic filling rate as a result of prolonged relaxation, which results in left atrial overload and pulmonary venous hypertension. Arterial stiffness increases with age, resulting in increased afterload and load-dependent diastolic dysfunction. In addition, stiffening of the central arteries (which is especially common in women) leaves them less capable to handle changes in blood volume, thereby increasing susceptibility to hypotension, lightheadedness, and dizziness. Finally, aging reduces exercise capacity by increasing ventricular end-systolic chamber elastance (stiffness), which results in decreased ability to augment contractility with exercise.
2. Hypertension—Hypertension is the most important risk factor for HFpEF and is present in most patients with HFpEF. Hypertensive emergency with flash pulmonary edema is a common presentation of HFpEF. Hypertension leads to LV hypertrophy, which causes impaired relaxation, poor coronary flow reserve, and increased diastolic stiffness, all of which exacerbate HFpEF. Hypertension is also a potent risk factor for epicardial coronary disease, which often complicates HFpEF. Ischemia causes both increased LV stiffness and impaired LV relaxation. Many patients with HFpEF have symptoms of chronic angina. Alternatively, recurrent HF may be an anginal equivalent in many patients with concomitant HFpEF and coronary disease.
3. Obstructive sleep apnea—Obstructive sleep apnea is a common comorbidity in patients with HFpEF, and it can result in worsening LV hypertrophy and pulmonary hypertension. In addition, patients with HFpEF may also have sleep-disordered breathing (such as Cheyne-Stokes respirations) due to their HF. Finally, increased upper airway edema due to generalized HF may actually cause obstructive sleep apnea, a finding that has been shown to improve with diuretic therapy. All of the above contribute to nocturnal microarousals and hypoxia, which result in poor sleep quality, which in turn worsens daytime fatigue and exercise intolerance. Therefore, there should be a low threshold to perform overnight polysomnography on the patient with HFpEF.
4. Other clinically important risk factors—Other clinically important risk factors for HFpEF include coronary artery disease, diabetes, chronic kidney disease, obesity, atrial fibrillation, anemia, and chronic obstructive pulmonary disease. All of these comorbidities have their own signs and symptoms that can complicate presentations of HFpEF and add to diagnostic, prognostic, and therapeutic complexities.
B. Symptoms & Signs
Symptoms and signs of HFpEF are identical to those in patients with HFrEF (systolic HF) and include dyspnea, fatigue, peripheral pitting edema, and jugular vein distention (see Chapter 26). Exercise intolerance and acute decompen-sated HF are two common presentations of HFpEF.
1. Exercise intolerance—Exercise intolerance is one of the main symptoms of HFpEF and one of the most debilitating. In patients with HFpEF, there are many reasons for exercise intolerance, including the following:
• Almost all patients with HFpEF have increased LV diastolic or left atrial pressures, or both. These pressure increases are transmitted to the pulmonary veins, which can cause decreased lung compliance, which is exacerbated by exercise.
• Increased LV diastolic pressure during exercise can limit subendocardial blood flow at a time when there are increased myocardial demands, thereby worsening diastolic function. Poor myocardial perfusion is even worse in patients with LV hypertrophy, which is very common in patients with HFpEF.
• Patients with HFpEF have an abnormal stroke volume response to tachycardia with blunted increase in cardiac output with exercise. Inadequate cardiac output can increase lactate production and worsen muscle fatigue.
2. Acutely decompensated HFpEF—The most common factor in acute decompensation is uncontrolled, severe hypertension. Other common clinical findings associated with acute decompensated HFpEF include arrhythmias; noncompliance with medications or salt restriction, or both; acute coronary syndrome; renal insufficiency; valvular regurgitation or stenosis; and infection (eg, pneumonia, urinary tract infection). It is important to recognize the clinical factors associated with acute decompensation because preventing hospitalization is one of the most important goals in patients with HFpEF.
C. Diagnostic Studies
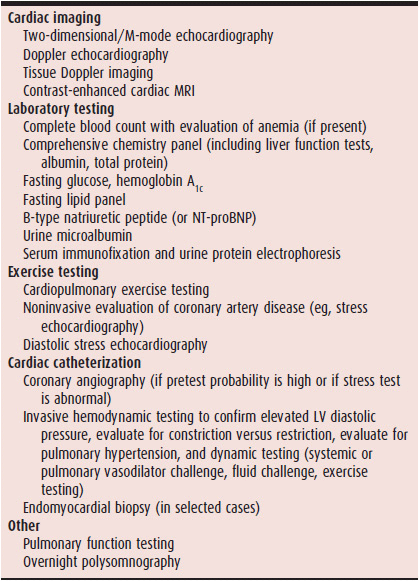
The diagnosis of HFpEF involves two steps: (1) making sure the patient has the HF syndrome (ie, evidence of elevated left-sided filling pressures) and a preserved ejection fraction (ie, LV ejection fraction ≥ 50%); and (2) determining the underlying cause of HFpEF once it is diagnosed. Echocardiography is a key diagnostic test because it allows the determination of LV ejection fraction, cardiac structural and functional abnormalities, and the comprehensive assessment of LV diastolic function, which can help rule in the presence of HF. Table 27–1 lists a standardized battery of diagnostic and prognostic tests for patients being evaluated for HFpEF.
Table 27–1. Diagnostic Evaluation of Heart Failure with Preserved Ejection Fraction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


