CHAPTER 117 Ventricular Septal Defect and Double-Outlet Right Ventricle
VENTRICULAR SEPTAL DEFECT
Banding of the pulmonary artery as a palliative maneuver was first described in 1952.1 This decreased left-to-right shunting and as a consequence prevented the development of pulmonary vascular obstructive disease and left-sided volume overload. Until the mid 1960s when primary VSD closure became safer, pulmonary artery banding was the procedure of choice in managing VSDs. The first VSD closure was performed in 1954 by Lillehei and associates2 at the University of Minnesota, using controlled cross-circulation between the child and parent. Nineteen of the 27 patients who underwent this procedure survived. In 1955, Kirklin and associates3 at the Mayo Clinic closed a VSD using a heart–lung machine. In 1958, transatrial VSD closure was performed,4 followed in 1969 by the popularization of primary repair in symptomatic infants by Barratt-Boyes and associates5 using cardiopulmonary bypass, deep hypothermia, and circulatory arrest.
Anatomy
Anatomy of the Tricuspid Valve, Right Ventricular Septum, and Conduction System
The right ventricular septum has five components (Fig. 117-1):
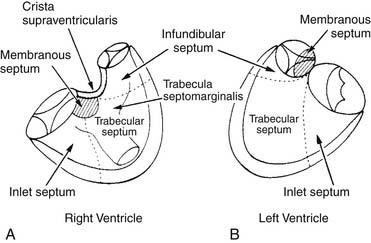
Figure 117–1 The components of the ventricular septum as seen from the right ventricle (A) and the left ventricle (B).
(Modified from Soto B, Becker AE, Moulaert AJ, et al. Br Heart J 1980;43: 332-7.)
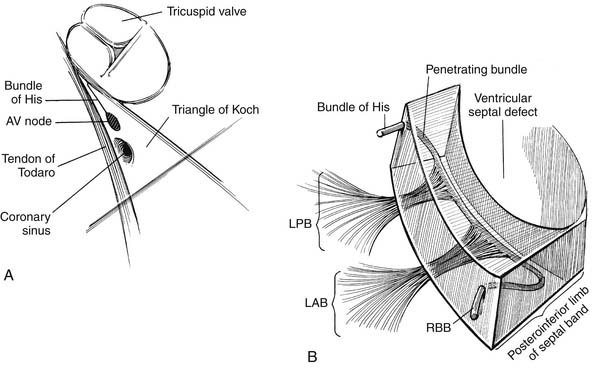
Knowledge of the conduction system of the heart also is critical when approaching VSDs so as to avoid damaging it (Fig. 117-2). The various atrial conduction tracts all converge toward the AV node of Aschoff-Tawara. The AV node is located in the inferior-posterior portion of the membranous septum, just inferior to the anteroseptal commissure of the tricuspid valve. A different description of its location is that it occupies the apex of the triangle of Koch, which is limited by the ligament of Todaro posteriorly, the orifice of the coronary sinus inferiorly, and the tricuspid valve annulus superiorly (see Fig. 117-2). From the AV node, the common AV bundle of His descends within the interventricular part of the membranous septum (or, in the case of a membranous VSD, the posteroinferior rim of the VSD), traverses the septum, and then courses along the left ventricular aspect of the septum. It then separates into a right bundle branch, which travels back to the right ventricular surface, as well as a left bundle branch. At the anteroinferior border at the level of the muscle of Lancisi, the right bundle branch descends toward the right ventricular apex.
Anatomic Classification of Ventricular Septal Defects
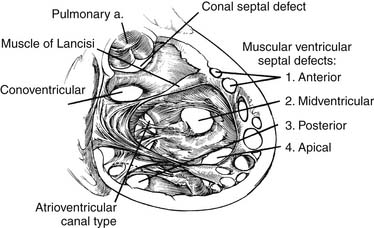
A useful surgical classification of VSDs was initially developed in 1980 by Soto and associates6 (Fig. 117-3) and then further modified by Van Praagh and associates (Fig. 117-4).7 Variations of this classification are used in most pediatric cardiac centers. VSDs can be classified as follows:
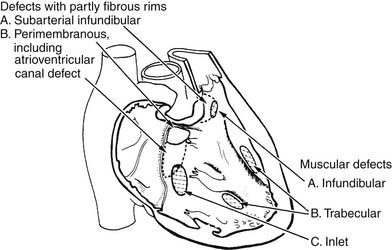
Figure 117–3 Classification of ventricular septal defects (VSDs) according to their location in the septum.
(Modified from Soto B, Becker AE, Moulaert AJ, et al. Br Heart J 1980;43: 332-7.)
Commonly Associated Defects
A large patent ductus arteriosus (PDA) is present in about 25% of symptomatic neonates or infants with VSDs.5 This is important to know because preoperative echocardiography may fail to show a PDA in the presence of a large amount of left-to-right shunting. Furthermore, intraoperative transesophageal echocardiography (TEE) is notoriously unreliable in excluding PDAs. Therefore the possibility of a PDA should be kept in mind when approaching a VSD, and if there is any doubt, or if there is a large amount of backflow through the pulmonary arteries on cardiopulmonary bypass, the PDA should be ligated or clipped.
A hemodynamically significant aortic coarctation is present in approximately 10% of cases. Because of the unique pathophysiology here (more left-to-right shunting across the VSD because of increased afterload caused by the coarctation), these patients usually have presenting symptoms before 3 months of age.8
Congenital valvar or subvalvar aortic stenosis, resulting in left ventricular outflow tract obstruction, is seen in approximately 4% of patients requiring an operation for VSD.9 The most common type of subaortic stenosis associated with VSDs involves the discrete fibromuscular membrane of the VSD that is located inferior or upstream to it. Congenital mitral valve stenosis is rare and occurs in about 2% of patients.
Pathophysiology
Pulmonary Vascular Disease
The classic description of the pathology of hypertensive pulmonary vascular disease is that of Heath and Edwards.10 They correlated the PVR of patients with large VSDs with the histologic severity of pulmonary vascular changes. Grade 1 changes were defined as medial hypertrophy without intimal proliferation; grade 2 as medial hypertrophy with cellular intimal reaction; grade 3 as intimal fibrosis and medial hypertrophy; grade 4 as generalized vascular dilation, an area of vascular occlusion by intimal fibrosis, and plexiform lesions; grade 5 as other “dilatation lesions” such as cavernous and angiomatoid lesions; and grade 6 as necrotizing arteritis. It is assumed that Heath-Edwards grade 3 or greater is not reversible. The importance of lung biopsies has decreased over the years, with catheterization-based data increasing in importance in terms of suitability for repair.
Natural History and Indications for Surgery
Approximately 30% of infants with severe symptoms such as intractable congestive heart failure or failure to thrive require surgery within the first year of life.11 The remainder can usually be managed medically, because the natural history of VSDs is well known.12 Aggressive medical management is indicated because a majority of membranous and muscular VSDs tend to close spontaneously.12 Malalignment conoventricular VSDs or inlet-type VSDs are unlikely to close spontaneously, and therefore closure at the time of diagnosis is recommended, regardless of age or weight. Asymptomatic children with isolated small restrictive VSDs can be followed safely with serial echocardiograms.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree