CHAPTER 115 Surgical Considerations in Pulmonary Vein Anomalies
ETIOLOGY
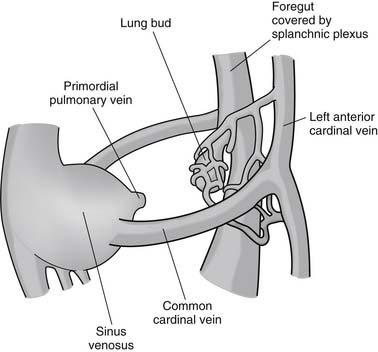
As part of normal embryologic development, the lungs are derived from buds arising from the primitive foregut. During the initial stages of pulmonary development (at 25 to 27 days of gestation), the pulmonary venous drainage is through the cardinal and umbilical-vitelline venous system (systemic veins). During the later stages of development (at 27 to 29 days of gestation), an outpouching of the common atrium forms to the leftward of the developing septal primum.1 The structure, termed the common pulmonary vein, primordial pulmonary vein, or pulmonary pit, extends and bifurcates into the pulmonary venous plexus and establishes venous drainage of the developing lung buds at 28 to 30 days of gestation (Fig. 115-1). Thereafter, the connection between the lung buds and the systemic venous system regresses, leaving the developing lungs with direct drainage to the left atrium.
PARTIAL ANOMALOUS PULMONARY VENOUS DRAINAGE
Anatomy
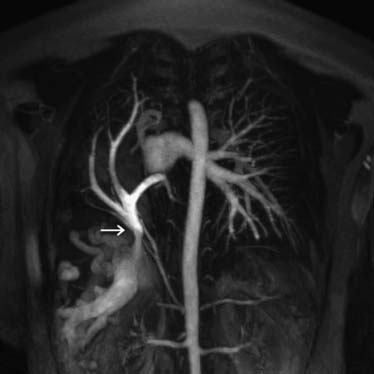
Partial anomalous pulmonary venous drainage is characterized by a failure of one to three of the pulmonary veins to connect with the left atrium during fetal development. Typically, the pulmonary vein has an abnormal connection to the superior vena cava near the cavoatrial junction. More than 80% of patients have associated congenital heart defects, the most common of which is an atrial septal defect. A minority of patients (18%) have an intact atrial septum.2,3 Most commonly, a combination of right upper and middle lobe pulmonary veins drain to the superior vena caval–right atrial junction. Less commonly, isolated anomalous right upper lobe venous drainage is present (Fig. 115-2). Least commonly, the entire right lung drains via an anomalous vein into the right atrium.3 Other less common sites of anomalous venous drainage include the inferior vena cava (e.g., as part of the scimitar syndrome), the left-sided superior vena cava, and the innominate vein.
Planning the Superior Vena Caval Cannulation
Careful review of the anatomy by preoperative echocardiography, computed tomography, or magnetic resonance imaging4 is the single most important component in developing an operative plan. Definition of the pulmonary venous anatomy and associated defects allows assessment of options for cannulation techniques and surgical approaches. This is especially important in preparation for partial anomalous pulmonary venous drainage, because the location of the anomalous pulmonary venous drainage determines the feasibility of a minimally invasive sternotomy incision and placement of venous cannulas. A common clinical scenario involves an anomalous right upper pulmonary vein inserting high into the superior vena cava, at or above the level of the pulmonary artery. Options for control of the superior vena cava include a high cannulation of the superior vena cava, cannulation of the innominate vein, or percutaneous cannulation via the internal jugular vein with vacuum-assisted venous drainage. A minimally invasive approach through a lower midline partial sternal split can be difficult when a high insertion of an anomalous pulmonary vein is present. A final technique to consider is the use of a straight venous cannula in the right atrial appendage, which can be directed into the superior vena cava. After snaring the cava around the cannula (cephalad to the anomalous pulmonary vein insertion), an atrial incision allows access to the origin of the anomalous pulmonary vein from within the atrium. Although this technique is easily accomplished with a minimally invasive lower midline partial sternal split, working around the cannula through the right atrial–superior vena caval junction can be challenging.
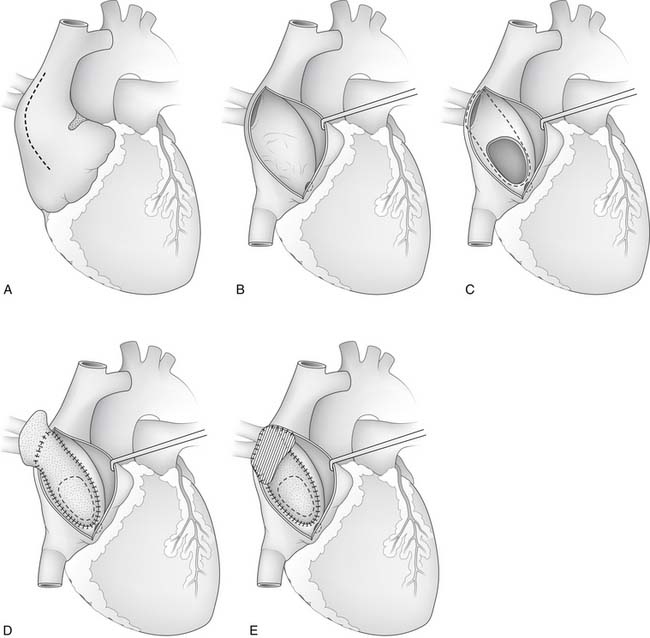
After heparinization, cannulation, and initiation of normothermic CPB, the aorta is clamped and blood cardioplegic arrest is obtained. It often is helpful to place a vent into the left atrium for later de-airing. A lateral right atriotomy is made. and the atrium is explored to identify any additional anomalies (Fig. 115-3). If the atrial septum is intact, an atrial septal defect is created by incising the region of the foramen ovale and extending the incision across the limbus in the direction of the anomalous pulmonary vein. Resection in the region of the limbus allows enlargement of the newly created atrial septal defect. Injury to the sinus node artery can occur when creating the atrial septal defect in patients whose artery courses through the superior portion of the intra-atrial septum. Enlargement of the sinus venosus defect might be necessary if it is too small to divert the anomalous pulmonary veins to the left atrium. Typically, a glutaraldehyde-treated pericardial patch can be used to create a baffle, so that pulmonary venous blood flows beneath the baffle and through the atrial septal defect into the left atrium.
Alternatively, a new anastomosis between the superior vena cava and the right atrial appendage is established by dividing the superior vena cava cephalad to the anomalous pulmonary venous entry and translocating the cephalad end of the superior vena cava to the right atrial appendage (Warden technique). The divided cardiac end of the superior vena cava (bearing the anomalous pulmonary venous connection) is closed, and a baffle is created from the orifice of the superior vena cava to the newly created atrial septal defect.3,5 By creating a new superior vena caval–right atrial junction, this procedure eliminates the need to partition the superior vena cava with a baffle dividing the systemic and pulmonary venous flow paths. This approach may be particularly helpful in infants and small children with a high insertion of an anomalous pulmonary vein into the superior vena cava.
Results
Early and long-term outcomes for patients after repair are excellent. In children, closure of an associated atrial septal defect almost eliminates the risk of late development of atrial arrhythmias. However, in adults, closure of the atrial septal defect is associated with persistent risk of development of atrial arrhythmias.6 Although extrapolation of these data for atrial septal defect closure to patients with partial anomalous pulmonary venous drainage and intact atrial septum has not been specifically validated, it seems reasonable to conclude that early removal of a left-to-right shunt associated with right ventricular overload is appropriate. In fact, surgical closure of the sinus venosus atrial septal defect at an older age is associated with increased risk of mortality, adverse events, and poor functional outcomes.7
Pertinent complications after repair of partial anomalous pulmonary venous drainage include stenosis of the superior vena cava or the anomalous pulmonary vein,8,9 residual atrial septal defects, and atrial arrhythmias or sinus node dysfunction (or both).9,10 Failure to include all pulmonary veins in the newly constructed baffle to the left atrium can result in a residual left-to-right shunt. However, incorporation of a systemic vein into the newly constructed baffle results in a residual right-to-left shunt. Finally, injury to the sinus node or sinus node artery can lead to the requirement for pacemaker insertion in patients with severe sinus node dysfunction.10
SCIMITAR SYNDROME
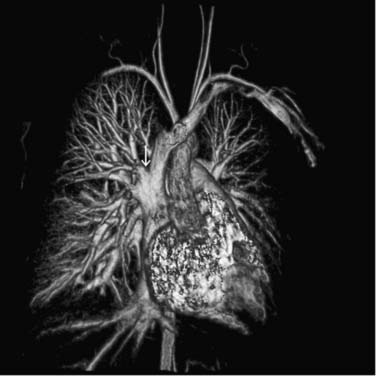
Scimitar syndrome is an unusual congenital anomaly that is manifested by partial anomalous pulmonary venous drainage of the right lung to the inferior vena cava and is often associated with hypoplasia of the right lung, dextrocardia, systemic pulmonary arterial supply from the abdominal aorta to the lower lobe of the right lung, and bronchial abnormalities. Other commonly associated anomalies include atrial septal defect, aortic coarctation, and a left-sided superior vena cava.11,12 The morphology of the anomalous pulmonary venous drainage to the right lower lobe creates a characteristic appearance to the right-sided heart border suggestive of a Turkish sword, hence the term scimitar syndrome (Fig. 115-4). The right-sided pulmonary veins drain to the inferior portions of the right atrium, the inferior vena caval–right atrial junction, or, more commonly, the inferior vena cava below the diaphragm. Stenosis may occur in 10% to 20% of scimitar veins at their junction to the inferior vena cava.13
The presentation of patients with scimitar syndrome can be variable and depends on the severity of the associated lesions. There are generally two forms of clinical presentation in scimitar syndrome: an infantile form with significant symptoms, morbidity, and mortality, and a form with mild symptoms seen in children or adults. At the most benign end of the spectrum, patients can display an asymptomatic flow murmur on physical examination as a result of increased pulmonary blood flow secondary to the left-to-right shunt caused by the anomalous pulmonary venous drainage to the inferior vena cava. At the most severe end of the spectrum, infants can present with symptoms of severe congestive heart failure, failure to thrive, tachypnea, and occasionally cyanosis. Infantile pulmonary artery pressures are often elevated (e.g., >40% of the systemic pressure), with a ratio of pulmonary blood flow (Qp) to systemic blood flow (Qs), or Qp/Qs, of up to 2:1.11
Surgical Management
The management strategy for patients with scimitar syndrome is determined by the degree of right lung hypoplasia, the presence of aortopulmonary sources of blood flow to the right lung, and the location and pattern of the right-sided pulmonary venous drainage. The degree of right lung hypoplasia is an important determinant of the likelihood of salvaging a functional right lung, and in cases of severe hypoplasia, a right pneumonectomy has been advocated.14 In cases with less severe hypoplasia, attention is turned to correction of the pulmonary venous drainage patterns, control of aortopulmonary sources of blood flow, and correction of associated anomalies. Control of the aortopulmonary sources of blood flow can be achieved through coil embolization in the catheterization laboratory, or by ligation in the operating room.15
Surgical repair of the pulmonary venous drainage is designed to create unobstructed flow from the anomalous pulmonary vein to the left atrium. To accomplish this, a direct surgical approach is to place a long baffle in the lumen of the inferior vena cava to channel the anomalous pulmonary vein effluent to the right atrium, and then through an atrial septal defect to the left atrium (originally described by Zubiate and Kay in 1962).16 It can be challenging to create an unobstructed baffle when the anomalous pulmonary venous connection is relatively caudad in the inferior vena cava. To construct a partitioning baffle, a period of circulatory arrest is typically necessary to secure a baffle between the pulmonary vein orifice and left atrium that allows unobstructed pulmonary vein flow without restricting flow in the inferior vena cava or in nearby hepatic veins.
When partitioning the inferior vena cava between the systemic and pulmonary venous flow paths, a right atrial incision can be carried down to the level of the anomalous pulmonary vein orifice, and the orifice is augmented with a patch of autologous pericardium if stenosis is present. The baffle is then constructed in the lumen of the inferior vena cava, and, if necessary, the inferior vena cava diameter can be augmented with a patch to ensure that there is no residual obstruction in the vena cava. This technique has a relatively high incidence of late stenosis, presumably because of the length of the intracaval baffle and because the blood must pass through a nearly 180-degree turn as it travels caudad toward the inferior vena cava and then cephalad up through the baffle to the atrial septal defect.14
A second approach is to divide the anomalous pulmonary vein and reimplant it at a convenient location in the right atrium and then create a baffle to divert flow to the left atrium through an atrial septal incision or atrial septal defect if present.17 This approach provides a shorter, more direct pathway for the pulmonary venous effluent. It is difficult to use this approach, however, when the anomalous pulmonary vein courses in the posterior portion of the lung and necessitates a great deal of augmentation to create an anastomosis with the atrium. When the pulmonary vein passes through the posterior mediastinum and the right lung is relatively hypoplastic, Huddleston and associates14 recommend pneumonectomy because of the high incidence of late stenosis with reimplantation techniques. As further justification of pneumonectomy in this setting, Huddleston and colleagues point out that the left lung has typically undergone some compensatory growth. Furthermore, perfusion scans typically demonstrate diminished pulmonary blood flow to the right lung in patients with scimitar syndrome.11 Calhoun and Mee described an alternative surgical approach for this entity, in which the inferior vena cava is transected under deep hypothermic circulatory arrest and divided into posterior (the anomalous pulmonary vein channel) and anterior (inferior vena cava and hepatic vein) compartments with a pericardial patch.18 An incision is made on the inferior aspect of the left atrium and carried to the atrial septum. The posterior compartment of the inferior vena cava is sutured to the left atriotomy and the pericardial patch is used to close the atrial septal defect. The anterior compartment of the inferior vena cava is anastomosed to the right atrium. This approach connects the anomalous pulmonary vein to the left atrium at a relatively gentle angle. Brown and coworkers reported favorable results with direct anastomosis of the anomalous pulmonary vein to the left atrium via right thoracotomy without the use of CPB.13
Results and Complications
Survival after complete repair (embolization or ligation of anomalous pulmonary arterial flow with correction of the anomalous pulmonary venous return) is anticipated in older children and adults. In recent series, 5-year survival rates have reached 100%, although a significant incidence of late pulmonary vein obstruction remains. In the infantile form of the lesion, however, hospital mortality is higher and depends on the degree of lung hypoplasia and the presence of pulmonary hypertension.11 Even with a technically perfect repair, blood flow to the right lung often remains diminished. The left-to-right shunt, however, is eliminated. Pulmonary venous obstruction is a prevalent late finding in many series and may be related to the surgical techniques used. Long pulmonary venous pathways through the inferior vena cava with acute angulation may predispose to stenosis. Recent reports using direct reimplantation techniques have demonstrated minimal rates of postoperative stenosis in small series of patients.13 Pneumonectomy remains an option in patients with persistently compromised pulmonary blood flow resulting from pulmonary hypertension or pulmonary venous obstruction.12,14
TOTAL ANOMALOUS PULMONARY VENOUS DRAINAGE
Total anomalous pulmonary venous drainage is a condition in which all the pulmonary venous effluent from the lungs drains to the systemic venous system, creating a large left-to-right shunt. This entity accounts for 1% to 3% of all congenital heart malformations.19 A right-to-left shunt must be present to allow blood to reach the left ventricle and thereby contribute to systemic cardiac output. Commonly, this shunt is at the atrial level as an atrial septal defect or patent foramen ovale. Less commonly, the shunt may be present as a ventricular septal defect or a patent ductus arteriosus. The absence of this shunt is incompatible with survival, and the magnitude of this shunt determines the systemic cardiac output.
Anatomy
Although well palliated in utero, infants with total anomalous pulmonary venous drainage have abnormalities in the pulmonary vascular system. There is often hypertrophy of the media of the pulmonary veins and arteries, intimal fibrous thickening of the pulmonary veins, and lymphangiectasia. These findings are accentuated in patients with pulmonary hypertension and evidence of pulmonary venous obstruction.20,21 The various types of total anomalous pulmonary venous drainage are classified by the site of connection to the systemic venous system.
Supracardiac Anatomy
Supracardiac drainage is the most common anatomic variant, occurring in 45% to 55% of patients with total anomalous pulmonary venous drainage.22,23 The venous connection is typically through a pulmonary venous confluence behind the left atrium to a connecting vein (often termed a vertical vein) to the innominate vein. Other sites of pulmonary–systemic connection can be to a left- or right-sided superior vena cava.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree