CHAPTER 14 Lung Transplantation
The first successful human lung transplantation was performed in 1983, by the Toronto Lung Transplant Group.1 More than 25 years have passed since this landmark procedure and more than 15,000 lung transplants have been performed. Lung transplantation is now the preferred treatment option for a variety of end-stage pulmonary diseases. Remarkable progress has occurred through refinements in technique and improved understanding of transplant immunology and microbiology. Despite these improvements, donor shortages and chronic lung allograft rejection continue to prevent pulmonary transplantation from reaching its full potential. Attempts made to address these issues include using marginal donors and living-lobar donors, split-lung donor techniques, and non-heart-beating donors.
HISTORICAL ASPECTS
In 1947, Vladimir Demikhov performed the first lung transplantation in a dog.2,3 The animal survived 7 days, dying from complications of bronchial dehiscence. Dr. James D. Hardy and colleagues performed the first human lung transplantation in 1963.4 Although their patient succumbed after 18 days, their brief success demonstrated the technical feasibility of the operation and stimulated worldwide interest in pulmonary transplantation. Over the next 20 years, approximately 40 lung transplants were attempted, but none achieved long-term success.5 The only recipient actually discharged from the hospital was a 23-year-old patient of Derom and colleagues,6 who left the hospital 8 months after transplantation and died a short time later as a result of chronic rejection, sepsis, and bronchial stenosis. Most patients in this era died within 2 weeks of lung transplantation as a result of primary graft failure, sepsis, or rejection. The most frequent cause of death beyond the second week was bronchial anastomotic disruption.
The Toronto Lung Transplant Group’s initial attempt at lung transplantation, in 1978, ended with the patient dying after a bronchial dehiscence.7 Through experimental studies, this group discovered that high-dose perioperative steroid usage contributed significantly to poor bronchial anastomotic healing,8 and that wrapping an omental pedicle around the bronchus resulted in restoration of blood supply and protection from dehiscence.9 Recipient selection was also addressed, as most of the early attempts had been in acutely ill, often ventilator-dependent patients. The Toronto group reasoned that end-stage respiratory failure from pulmonary fibrosis would provide the ideal physiologic conditions for single-lung transplantation. The increased resistance to both perfusion and ventilation of the native lung would preferentially direct perfusion and ventilation to the transplanted lung. On November 7, 1983, the first successful isolated lung transplant was performed in a 58-year-old man with pulmonary fibrosis.1 The use of the omental flap and withholding steroids perioperatively are now mainly of historical interest. In the final analysis, it appears to be the attention to detail that this group practiced that led to long-term success.
Patterson and colleagues used the en bloc double-lung transplant technique to extend the use of lung transplantation to patients with septic lung diseases and emphysema.10 The en bloc double-lung transplant, however, had several drawbacks: it was technically difficult, it required cardiopulmonary bypass (CPB), tracheal anastomotic ischemic complications were frequent, cardiac denervation occurred, and bleeding into the posterior mediastinum was troublesome because of poor operative exposure. To circumvent these problems, Pasque and colleagues11 devised the technique of bilateral sequential pulmonary transplantation. A transverse thoracosternotomy provided excellent exposure of the mediastinum and both pleural spaces. Recently, this incision was modified to avoid sternal division, when possible, to eliminate sternal wound complications.
Patients with emphysema were initially felt to be poor candidates for single-lung transplantation, because the overly compliant native lung would be prone to hyperinflation, resulting in mediastinal shift and compression of the transplanted lung. Despite this concern, successful single-lung transplantation for emphysema was reported in 1989 by Mal and colleagues.12 Pasque and colleagues13 reported success with single-lung transplantation for patients with pulmonary hypertension. The first applications of living related lobar transplants were reported by Starnes and colleagues.14
Split-lung techniques have been developed to allow a large left donor lung to be bipartitioned and the individual lobes transplanted bilaterally.15 Furthermore, the use of lungs from non-heart-beating donors has moved from the research realm to clinical reality.16
SELECTION
Recipients
General selection criteria are listed in Box 14-1 and are summarized by Maurer and colleagues.17
Patients with failure of another organ system and patients over the age of 65 are generally not eligible for transplantation. Advanced recipient age is a specific predictor of increased post-transplant mortality.18 Additionally, a history of malignant disease within the prior 5 years generally precludes pulmonary transplantation. A potential exception is a patient with bilateral bronchoalveolar carcinoma. Etienne and colleagues19 have reported a single long-term survivor with this therapy, and Zorn and associates20 have reported a favorable experience in a small number of patients. The patient with a recent extrathoracic malignancy judged to be cured might be considered. Patients with serious psychological dysfunction should not be considered candidates for pulmonary transplantation. Few groups evaluate patients who continue to smoke.
Previous thoracic surgery is not a specific contraindication to pulmonary transplantation, although it may complicate a subsequent transplant procedure. Patients receiving high-dose corticosteroid therapy (≥20 mg prednisone) are not eligible for lung transplantation, as a well-documented negative influence on bronchial healing and susceptibility to postoperative infection has been demonstrated. However, low- or moderate-dose steroid therapy does not result in an increased incidence of bronchial anastomotic complications. Ventilator dependency is not a specific contraindication to transplantation, but it has been identified as a risk factor for increased mortality.18
Lung allocation in the United States was previously based on time on the waitlist, regardless of medical urgency or deterioration in medical condition. This system was flawed as it favored recipients well enough to survive on the transplant list, whereas those who might benefit most risked death while waiting. An ideal system of organ allocation balances clinical necessity with ability to recover from a transplant operation. Recently, the United Network for Organ Sharing (UNOS) Thoracic Organ Committee revised the listing algorithm by assigning each patient a lung allocation score (LAS) based on the immediate need for transplant and the probability of post-transplant survival. This led the UNOS, in 2005, to rearrange its waitlist for adult lung transplantation to one based on the LAS score. Details of the new allocation system are found on the official Organ Procurement and Transplantation Network website.21
The LAS score is calculated by estimating waitlist urgency, defined as the expected number of days that could be lived without a transplant (Box 14-2), and post-transplantation survival, defined as the expected number of days lived during the first year after transplantation (Box 14-3). The transplant benefit measure is then derived by subtracting the waitlist urgency from the post-transplantation survival to obtain the raw allocation score (calculated in days). This score is normalized to the LAS on a scale of 1 to 100. The highest scores are listed first for transplantation. Factors used to predict risk of death and post-transplantation survival are regularly reviewed by the Thoracic Organ Transplantation Committee and updated as appropriate.
Box 14–2 Factors That Predict Risk of Death for Patients on the Transplant Waiting List
From Organ Procurement and Transplant Network, available at http://optn.transplant.hrsa.gov/PoliciesandBylaws2/policies/pdfs/policy_9.pdf
Disease-Specific Guidelines
Obstructive lung disease, notably emphysema and α1-antitrypsin deficiency, has previously been the most common indication for lung transplantation, accounting for 46% of the adult lung transplantations reported in the 2007 Registry of UNOS and the International Society for Heart and Lung Transplantation (ISHLT).18
Before being considered for pulmonary transplantation, patients with obstructive lung disease should have maximization of medical therapy including bronchodilators and oxygen. Consideration should be given to lung volume reduction surgery (LVRS) in ideal patients (with hyperinflation, heterogeneous distribution of disease, forced expiratory volume in 1 second (FEV1) of more than 20%, and normal PCO2).22 We have not found LVRS to be an ideal option in patients with α1-antitrypsin deficiency, as in general these patients have diffuse disease.22 Preliminary LVRS does not jeopardize subsequent successful lung transplantation.23
We favor a meticulous selection process in which both transplantation and volume reduction are considered, and the best option is selected for each patient. In general, in patients with obstructive lung disease, the FEV1 should be less than 25% of predicted value, and not reversible, although most patients actually have an FEV1 of less than 15% of predicted at the time of transplantation. Progressive deterioration as evidenced by hypercarbia (PaCO2 ≥ 55 mm Hg), increasing oxygen requirement (resting PaO2 < 55 mm Hg), the development of secondary pulmonary hypertension, rapid decline of FEV1, or frequent life-threatening infections indicate decreased survival, suggesting the need for transplantation.17
Septic lung disease is another common indication for pulmonary transplantation. Cystic fibrosis (CF) is a common inherited disorder resulting in diffuse bronchiectatic destruction of both lungs. Without transplantation, the overwhelming majority of patients die as a result of progressive respiratory failure in the second or third decade of life. As reported in the 2007 ISHLT Registry, CF is now the most common indication for bilateral lung transplantation and the third most common indication for lung transplant in general.18 The most reliable predictors of life expectancy in CF patients were described by Kerem and associates.24 An FEV1 of less than 30% predicted, elevated PaCO2, requirement for supplemental oxygen, frequent admissions to the hospital for control of acute pulmonary infection, and failure to maintain weight are reliable predictors of early mortality in these patients. At this stage of disease, patients with CF usually have a rapidly progressive downhill course. To offset this high waitlist mortality, some of these listed factors have been incorporated into the LAS.
Patients with septic lung disease or those with significant sputum production are evaluated with frequent sputum cultures to assess bacterial sensitivities. Inhaled high-dose aminoglycosides or colistin are frequently used in this population. Patients with multidrug-resistant organisms, especially pan-resistant Burkholderia cepacia, are considered at high risk, and many centers consider this a contraindication to transplantation. Early mortality in patients with CF who have B. cepacia infection is significantly increased.25,26 Successful transplantation in these patients often requires the use of multiple combinations of intravenous antibiotics, after in vitro synergy testing to guide antibiotic selection. If no susceptible antibiotic regimen is found on synergy testing, we do not proceed with transplantation.
The diagnoses of pulmonary fibrosis and restrictive lung disease include idiopathic pulmonary fibrosis (IPF), pulmonary fibrosis of other etiologies, sarcoidosis with elevated pulmonary artery pressures, and obliterative bronchiolitis (not retransplant cases). The 2007 ISHLT Registry report indicated that IPF was the second most common indication for single-lung transplantation and the third most common for bilateral lung transplantation.18 In our experience, candidates for transplantation had classic restrictive findings on spirometry, with a mean forced vital capacity of 1.35 L and an FEV1 of 1.14 L.27 All used supplemental oxygen and demonstrated marked impairments in exercise tolerance. Moderate pulmonary hypertension is common in these patients. Patients with pulmonary fibrosis who require a transplant have been observed to have a rapid downhill course. The lung allocation score has been devised to recognize the medical urgency of these patients.
Previously, patients with pulmonary hypertension were considered for lung transplantation early in the course of their disease because of the poor outcome of this disease process. With the use of prostacyclins and other vasodilator therapies, such as endothelin receptor antagonists and phosphodiesterase inhibitors, an improvement in pulmonary artery pressures and relief of symptoms are seen in the majority of patients with primary pulmonary hypertension.28,29 Transplantation may be delayed as long as these patients remain clinically stable on vasodilatory therapy. For patients with pulmonary hypertension secondary to congenital heart defects or thromboembolic diseases, surgical intervention for the primary diagnosis should be considered. The current indication for transplantation is progressive deterioration despite optimal therapy (e.g., New York Heart Association [NYHA] class III or IV, mean pulmonary artery pressure >50, right atrial pressure >10 mm Hg, cardiac index <2.5 L/min/m2, syncopal episodes).17 We prefer bilateral lung transplantation in this group; if the patient is to undergo single-lung transplantation, we do not use a marginal donor lung, as the bulk of the cardiac output will be through the transplanted lung. Patients with Eisenmenger’s syndrome and secondary pulmonary hypertension have not shown an improvement in survival after lung transplantation.30,31
Donors
Rapid progress in the field of transplantation has resulted in a shortage of suitable allografts for all organs. This problem is particularly significant for lung transplantation, as only 20% of otherwise suitable organ donors have lungs satisfactory for transplantation, according to the criteria listed in Box 14-4. A majority of conditions resulting in brain death (e.g., trauma, spontaneous intracerebral hemorrhage) also lead to significant pulmonary parenchymal pathologic change because of lung contusion, infection, aspiration, or neurogenic pulmonary edema.
The donor’s medical history is obtained, with particular emphasis on and attention paid to the donor’s age, cause of death, timing of death, smoking history, and prior thoracic procedures. Older donor age (greater than 60) is considered a significant risk factor for adverse outcome after pulmonary transplantation, and in general donors younger than 55 are preferred.32 Although a significant smoking history (≥30 pack-years) in the donor is a concern, it is not an absolute contraindication to the use of otherwise suitable donor lungs. ABO incompatibility between donor and recipient, human immunodeficiency virus positivity, active malignancy (outside the central nervous system), and active hepatitis infection remain absolute contraindications to donor lung procurement. Histocompatibility matching for human leukocyte antigens (HLAs) is not usually performed between donor and recipient before transplantation unless the patient has an elevated panel reactive antibody or known HLA antibodies from prior sensitization.
Certain circumstances allow relaxation of the typically strict donor selection criteria. A minor degree of pulmonary infiltrate may be accepted in donor lungs being used for a bilateral transplantation. We analyzed 133 consecutive donor lungs and identified 37 with marginal quality, as judged by arterial blood gas analysis and radiographic assessment. The marginal donors provided postoperative function equivalent to those judged excellent.33
Novel techniques to optimize donor usage have been introduced. The first is a split-lung technique that bipartitions the left lung of a large cadaveric donor and uses the two lobes to perform a bilateral lobar transplant in the smaller recipient. It requires significant expertise in lung transplantation, but it has been performed successfully with good outcomes at certain institutions.15,34
Another technique is the use of non-heart-beating donors.16 The ability of pulmonary parenchymal cells to continue aerobic cellular metabolism by relying on the oxygen in the alveoli make the lung potentially the ideal organ for transplantation after cessation of circulation—so-called donation after cardiac death. A series from Spain reported excellent early allograft function with no ischemia-reperfusion injury even after 11 hours of total ischemia, and outpatient follow-up to 13 months after transplantation documented adequate lung function and quality of life.35 More recent follow-up of this experience demonstrates a 3-year survival of 58% in 17 recipients of lungs from non-heart-beating donors.36 Steen and colleagues from Sweden have advocated extracorporeal perfusion and ex vivo assessment of donor lung function in non-heart-beating donors and have described their initial clinical experience with ex vivo reconditioning of initially unacceptable lungs.37
DONOR SURGERY
Procurement
Brain-Dead Donor
The superior vena cava (SVC) is encircled caudal to the azygous vein with heavy silk suture. Encircling the inferior vena cava (IVC) is optional. The plane between the anterior surface of the right PA and the back of the SVC and the ascending aorta is developed. The aortopulmonary window is dissected, and the aorta is encircled with an umbilical tape: this is useful in the eventual placement of the aortic crossclamp when the pericardial sac is full of blood. The SVC and the aorta are gently retracted, and the posterior pericardium is incised above the right PA, allowing access to the trachea. The plane around the trachea is developed using finger dissection. Alternatively, the posterior mediastinal dissection, including dissection of the trachea, can be entirely performed after explanting the heart. After completion of the thoracic dissection and ensuring that abdominal organ procurement teams are ready, the donor is systemically heparinized (250 to 300 units/kg). The ascending aorta is cannulated with a routine cardioplegia cannula for cardiac preservation. A U-stitch is placed just proximal to the bifurcation of the main PA, and a Sarns (Sarns, Ann Arbor, MI) 6.5-mm curved metal cannula is placed into the main PA with the cannula tip pointing toward the PA bifurcation (Fig. 14-1).38
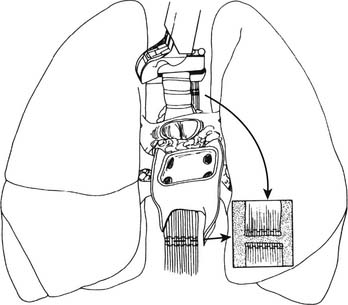
Using a fine needle, a 500-μg bolus dose of prostaglandin-E1 is injected directly into the PA. Hypotension should be expected with this maneuver. Next, the SVC is ligated after ensuring that there is no central venous catheter in the lumen and the IVC is divided, thus venting the right heart. The aorta is cross-clamped and cardioplegia initiated. The left atrial appendage is generously incised, venting the left side of the heart (see Fig. 14-1). The pulmonary preservation solution consisting of several liters (50 to 75 mL/kg) of cold (4° C) Perfadex is initiated via the PA cannula. Ice slush is generously used to topically cool the heart and both pleural spaces. Gentle ventilation is continued to prevent atelectasis and homogeneously distribute the perfusate. Clear perfusate exiting the left atriotomy confirms adequate lung flushing. After completion of the cardioplegia and the antegrade pulmonary flush, the cannulae are removed. The IVC is now freed posteriorly and dissected up to the level of the right atrium, ensuring that the right inferior pulmonary vein is not damaged during this maneuver. Division of the left atrium ensues with the cooperation of the heart and lung teams. The heart is retracted toward the right, and an incision is made with a #11-blade scalpel in the left atrium midway between the coronary sinus and the left inferior pulmonary vein. Scissors are then used to extend the opening superiorly and inferiorly while visualizing the orifices of the left superior and inferior pulmonary veins from inside the atrium. The remaining cuff of the left atrium is transected while visualizing the orifice of the right pulmonary veins from within the atrium. An appropriate residual atrial cuff should have a rim of left atrial muscle around each of the pulmonary vein orifices (Fig. 14-2).
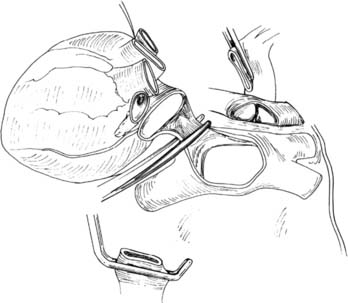
Figure 14–2 The heart is being explanted, leaving a rim of atrium around each pulmonary vein orifice.
(From Sundaresan S, Trachiotis GD, Aoe M, Patterson GA, Cooper JD. Ann Thorac Surg 1993;56:1409-13.)
The SVC is now transected between ties. This is followed by division of the aorta proximal to the crossclamp and the PA at the site of cannulation. The heart is then passed off the field. Next, we use a Foley catheter to deliver about 250 mL of retrograde pulmonary flush via each of the pulmonary vein orifices, which often delivers residual blood and small clots out of the open PA bifurcation. This retrograde flush may also be performed on the back table at the donor site. Retrograde pulmonary perfusion has been shown to lead to better oxygenation, higher compliance, and a lower extravascular lung water index in transplanted lungs in the experimental setting.39
We then proceed with en bloc removal of the mediastinal contents. This technique avoids injury to the membranous trachea, pulmonary arteries, and pulmonary veins, and it preserves maximal soft tissue for collateral flow to the airway. The superior mediastinal tissues, including the great vessels, are divided, and the trachea is encircled two to three rings above the carina. The endotracheal tube is opened to the atmosphere and the lungs are allowed to deflate to approximate end-tidal volume while the endotracheal tube is backed into the proximal trachea. The trachea is divided between staple lines at least two rings above the carina. The esophagus is also divided using a stapler (Fig. 14-3).
(From Sundaresan S, Trachiotis GD, Aoe M, Patterson GA, Cooper JD. Ann Thorac Surg 1993;56:1409-13.)
Non-Heart-Beating Donor
The mid-term results of the largest single clinical series of non-heart-beating donor lung transplantation have been published,36 and the technique of donor organ procurement is summarized here. After systemic heparinization, the donor is placed on arteriovenous extracorporeal membrane oxygenation (ECMO) via a femoral approach. A Fogarty catheter is placed in the supradiaphragmatic aorta for better abdominal organ perfusion, and bilateral chest tubes are placed for topical lung cooling with cold Perfadex. De Antonio and colleagues showed that the lung could tolerate a warm ischemia time of 120 minutes and cold preservation time (cooling to harvest) of up to 240 minutes. After obtaining consent, bronchoscopy is performed and the chest is opened. Ventilation is now resumed with 100% FiO2 and 5 cm of positive end-expiratory pressure (PEEP). The pleural spaces are drained and the pericardium is opened. The aorta is clamped and both venae cavae are ligated. Antegrade lung perfusion is performed with 5 to 6 L of Perfadex, followed by infusion of 300 mL of donor blood through the PA. A blood gas analysis is performed on the left atrial effluent. Retrograde perfusion and the lung procurement are now performed in routine fashion.36
Atrial Cuff and Pulmonary Vein Injuries
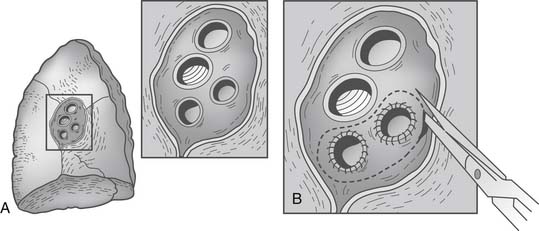
If donor left atrial cuff is inadequate or the superior and inferior veins are completely separated, a reconstructive salvage technique using donor pericardium has been described40 (Fig. 14-4). It is estimated that up to 2.7% of patients may need atrial cuff reconstruction, and Oto and colleagues have elegantly illustrated their techniques (Fig. 14-5).41
Congenital Bronchial Anomalies
A tracheal upper lobe bronchus, the most common anomaly, may be a segmental or a lobar bronchus. If the bronchus is determined to be a segmental bronchus, it may be simply oversewn or reimplanted. If the entire upper lobe bronchus arises as an abnormal tracheal bronchus, the options are donor right upper lobectomy, left single-lung transplantation, or incorporation of the bronchus intermedius and the aberrant upper lobe bronchus into a modified anastomosis with the recipient bronchus.42
RECIPIENT SURGERY
Anesthesia and Intraoperative Conduct
TEE is useful at various junctures in the operation.43 In particular, it is useful for TEE, monitoring for acute right ventricular dilation during PA clamping, detecting intracardiac shunts, determining the need for CPB based on classic signs of impending right ventricular decompensation, and assessment of the arterial and venous anastomoses and presence of air in the left atrium. We use epoprostenol or nitric oxide (or both) for acute refractory pulmonary hypertension in the perioperative period. Inhaled nitric oxide is also indicated for poor oxygenation.
Implantation
Incisions
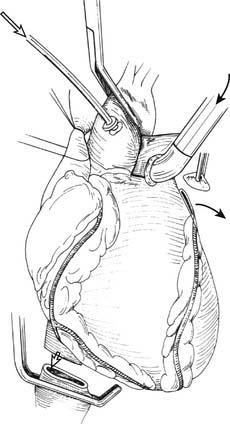
Bilateral anterolateral thoracotomy without sternal division is our preferred incision for bilateral sequential lung transplant.44 The skin incision follows the inframammary crease at the level of the fourth intercostal space and extends from the lateral sternal edge to the anterior axillary line. The breast tissue is elevated and the pectoralis major muscle is divided. The chest cavity is entered in the fourth interspace. Bilateral internal mammary arteries are ligated and divided. Alternatively, the internal mammary arteries can be preserved if a 1-cm segment of costal cartilage of the fourth rib is resected at the sternal border, allowing upward mobility of the fourth rib when retracted. Further mobility for retraction is obtained by dividing the intercostal muscles from within the pleural space laterally to the paraspinal muscles. We place two chest wall retractors at 90-degree angles to one another (Fig. 14-6). If needed, a right anterolateral thoracotomy allows adequate exposure of the aorta and right atrium for CPB.
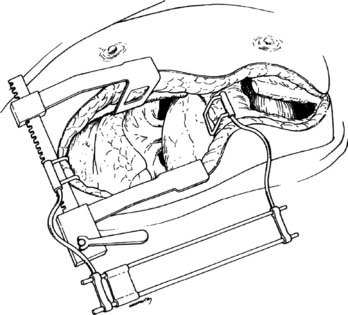
Figure 14–6 Bilateral anterolateral thoracotomy. Two retractors are placed at right angles.
(From Meyers BF, Patterson GA. Technical aspects of adult lung transplantation. Semin Thorac Cardiovasc Surg 1998;10:213-20.)
The clamshell incision (sternothoracotomy) involves connecting the bilateral anterolateral thoracotomy incisions across the midline by dividing the sternum (Fig. 14-7). This incision provides excellent exposure and requires the division of both mammary arteries. It is used for providing added exposure when a concomitant cardiac procedure is performed, or when cardiomegaly or a relatively small chest cavity makes hilar exposure difficult. The sternum is reapproximated using a heavy-gauge Steinmann pin and two figure-eight #5 sternal wires.
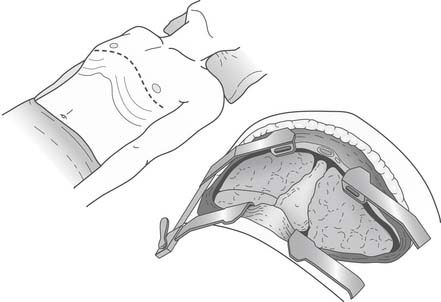
Figure 14–7 The sternum has been divided for a clamshell incision that provides excellent exposure to the thorax.
(From Lau CL, Patterson GA. Technical considerations in lung transplantation. Chest Surg Clin N Am 2003;13:463-483.)
The anterior axillary muscle-sparing thoracotomy incision, which may lead to improved chest wall and shoulder girdle mechanics, was initially described for single-lung transplant in recipients with chronic obstructive pulmonary disease.45 The small anteroaxillary thoracotomy has been found comparable with the more conventional posterolateral or clamshell incision in terms of operating times and ability to go on central CPB.46,47
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree