Chapter 55 Ventricular and Supraventricular Tachyarrhythmias Associated with Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a genetic cardiac disease with a particularly heterogeneous clinical presentation and diverse natural history.1–5 Sudden and unexpected death has been recognized as a prominent and devastating complication of HCM since the initial contemporary description of this disease over 50 years ago.6 Ventricular tachyarrhythmias have always represented a focus of concern in this disease because of an apparent causal relationship to sudden cardiac death (SCD).7–22 In addition, it is now evident that supraventricular tachyarrhythmias (particularly atrial fibrillation [AF]) commonly occur in HCM and can also represent important determinants of clinical course.23 This chapter discusses the linkage of tachyarrhythmias to SCD, disease progression in HCM, and the role of preventive interventions.
Ventricular Arrhythmias and Sudden Cardiac Death
Historical Context
Recognition that a small but important subgroup of patients with HCM is at increased risk for SCD has for many years generated considerable interest in the role of arrhythmias and the process of risk stratification.1,3,5,8–10,12,17,20,22 This focus is now particularly acute with the availability of implantable cardioverter defibrillators (ICDs) for the effective prevention of these unpredictable catastrophes.16,24 It has been emphasized that SCD in HCM usually occurs in young asymptomatic patients, with annual mortality rates initially reported as up to 4% to 6% in tertiary-center referral populations, which are disproportionately composed of high-risk patients, and more recently 1% per year in less selective community-based cohorts.3,5,1–22,25–27
On routine ambulatory (Holter) monitoring, more than 90% of HCM patients demonstrate frequent and complex ventricular tachyarrhythmias, including premature ventricular depolarizations or couplets and nonsustained ventricular tachycardia (VT) (Figure 55-1).7,8,10,12,19,20 Although the frequency of these ventricular arrhythmias appears disproportionate to the occurrence of SCD in HCM, short bursts of nonsustained VT were identified 25 years ago as markers for SCD risk, focusing attention at that time on 24-hour ambulatory (Holter) electrocardiogram (ECG) monitoring for occult arrhythmia detection before major adverse cardiac events, an observation that triggered an era of prophylactic anti-arrhythmic drug treatment.7,8,10,12,18–20
Nonsustained Ventricular Tachycardia
Ventricular arrhythmias in patients with HCM are largely clinically silent. Indeed, short runs of asymptomatic nonsustained VT commonly occur in 20% to 25% of HCM patients on 24-hour ambulatory ECG monitoring.7–9,11,12,17 Nonsustained VT confers about an eightfold increased risk for SCD—that is, 8% per year compared with 1% per year in the absence of VT, high negative predictive values, and low positive predictive values for events (i.e., 96% and 26%, respectively).11,12,18 These relatively low positive predictive values are largely attributable to the low event rate characteristic of HCM and similar to that of other risk markers in this disease.28
Nevertheless, nonsustained VT on ambulatory Holter monitoring as a risk factor has been fraught with particular ambiguity over the years.28–30 For example, a single brief isolated burst of nonsustained VT on a random Holter ECG is no longer regarded, per se, as a risk marker that should trigger ICD implantation for primary prevention.1,20 However, longer VT runs (>10 beats/min) intuitively carry greater weight in these clinical circumstances. One potentially useful (but non–evidence-based) strategy that may be considered following the identification of a short run of VT on the initial 24-hour Holter ECG involves a more extended ambulatory monitoring period of 7 to 21 days. This approach achieves a more measured assessment of an individual patient’s day-to-day ectopy profile, potentially influencing or clarifying decisions concerning prophylactic ICDs. Conversely, and of particular importance, the absence of VT on Holter monitoring in patients with otherwise low-risk clinical profiles dictates a large measure of reassurance with regard to prognosis.
Selection of Patients for Implantable Cardioverter-Defibrillators
Conventional Risk Markers
It is universally agreed that HCM patients should be afforded secondary prevention ICDs following cardiac arrest or sustained episodes of VT (with or without hemodynamic instability), including recommendations from the American College of Cardiology/European Society of Cardiology (ACC/ESC) expert consensus HCM panel.3,5 However, selection of patients most likely to benefit from prophylactic ICD therapy is less certain, with implantation guidelines being a long-evolving and sometimes contentious issue for which definitive resolution has been elusive.
Risk stratification in HCM is predicated on the assessment of noninvasive risk markers, usually in clinically stable patients, which have emerged from observational studies and achieved general acceptance.1,3,5,10–22,24 In this respect, the ICD strategy differs from that used in patients with coronary artery disease (CAD)—that is, with primary prevention being based largely on a single predominant risk marker emanating from a major clinical event (i.e., myocardial infarction [MI]), leading to left ventricular (LV) remodeling and impaired cardiac function with ejection fraction of 30% to 35% or less and frequently adverse disease progression.30–32
The acknowledged primary prevention HCM risk factors are: (1) family history of one or more HCM-related SCDs; (2) one or more episode of unexplained, recent syncope; (3) multiple, repetitive, or prolonged nonsustained VT on more than one ambulatory 24-hour ECG; (4) hypotensive or attenuated blood pressure response to exercise; (5) massive LV hypertrophy (wall thickness ≥30 mm on echocardiography) (see Figure 55-6). Each of these risk markers applies largely to patients younger than 50 years, and only one (the ambulatory ECG) explores the arrhythmogenic substrate.
Patients in other HCM subgroups who may also be selectively considered at increased risk are those with (1) LV apical aneurysm with regional scarring; (2) end-stage phase with systolic dysfunction; (3) transmural MI following alcohol septal ablation.33–35
One risk factor judged to be major in a given HCM patient can be sufficient to justify consideration for a primary prevention ICD.22,24,29 However, the presence of multiple risk factors certainly creates a clinical environment in which decision making regarding ICD implantation becomes much more intuitive and easier. Nevertheless, it should be underscored that not all patients with one risk factor require prophylactic implants.22,24,29 Indeed, numerous complex clinical scenarios exist in HCM, with ambiguities and gray areas that arise with respect to the presence, strength, and number of risk factors ultimately affecting those decisions regarding prophylaxis. One specific example in this regard would be older HCM patients with unexplained syncope as a single risk factor. Such patients may not be candidates for primary prevention, given that HCM-related SCD is uncommon in this age group, survival to advanced age itself determines the lower risk status in this disease, and syncope is not uncommon at advanced ages.24,29 However, syncope as a single risk factor necessitating primary prevention ICDs has been associated with appropriately high intervention rates in HCM.22,24,29 Finally, the available data do not permit assessment of the relative weight that each risk factor can be assigned in the individual patient.
Potential Mechanisms of Sudden Cardiac Death
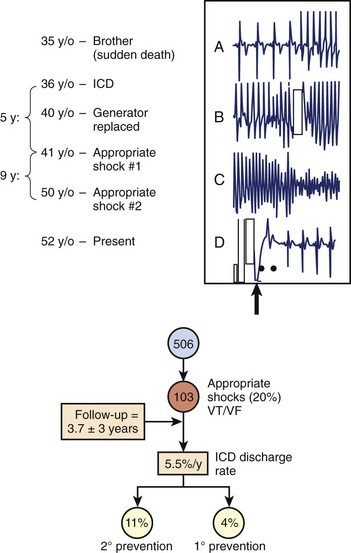
While the pathogenesis of SCD in HCM is likely complex and multi-factorial (and remains incompletely defined), such events emanate from primary ventricular tachyarrhythmias.1–24,36–38 These arrhythmia sequences have been documented by stored electrocardiographic recordings in patients with ICDs experiencing appropriate device interventions (Figure 55-2).16,22,24 These observations offer a unique window to understanding the mechanisms responsible for SCD in HCM. It has not been possible, however, to exclude bradycardia-mediated events conclusively because of the backup pacing capability operative in many of the devices, and it is possible that other more diverse arrhythmia mechanisms may ultimately prove to be involved.37,38 Complete heart block and accelerated atrioventricular (AV) conduction caused by accessory pathways are acknowledged, even though particularly rare, as causes of syncope or SCD in HCM.39
Ventricular tachyarrhythmias in HCM probably emanate primarily from a substrate of electrical instability and disordered electrophysiological transmission caused by disorganized left ventricular cellular architecture.6,40,41 Alternatively, they may be caused by bursts of myocardial ischemia (probably because of structurally abnormal, narrowed intramural arterioles), leading to myocyte necrosis and repair in the form of replacement fibrosis (Figure 55-3).42 This myocardial substrate may be vulnerable to a variety of triggers, either intrinsically—related to the HCM disease process—or to extrinsic environmental factors such as intense physical exertion (including in athletes). In addition, a variable and defined component of individual patient susceptibility undoubtedly plays a role in determining which HCM patients experience clinical events at particular moments in their lives.43
Prevention of Sudden Cardiac Death
Pharmacologic Treatment
Historically, the management of high-risk HCM patients was limited to prophylactic pharmacologic treatment with β-blockers, verapamil, antiarrhythmic agents such as procainamide and quinidine, and more recently amiodarone.44–46 However, no convincing data in HCM support the efficacy of prophylactic drug treatment for SCD.1,5 For example, no controlled studies address the effects of β-blockers or verapamil on SCD. Type IA antiarrhythmic agents (such as procainamide and quinidine) have long been abandoned in the treatment for isolated or infrequent bursts of nonsustained VT on ambulatory Holter monitoring in HCM patients because of the potential proarrhythmic effects of these drugs.12,44
Furthermore, the efficacy of amiodarone in preventing SCD in HCM has been called into question by observations in studies in which this drug did not offer complete protection against appropriate ICD shocks or cardiac arrest.16,22,24,46 Also, the frequent adverse consequences associated with the long-term administration of amiodarone greatly limit its application to SCD prevention in young patients with HCM who characteristically have long periods of risk.
Implantable Defibrillators
Since its introduction by Mirowski and Mower 30 years ago,47 the ICD has achieved widespread acceptance as a preventive treatment for SCD by virtue of the indisputable evidence of its efficacy in terminating life-threatening ventricular tachyarrhythmias and prolonging life, principally in high-risk patients with ischemic heart disease.30–32,47,48
In HCM, evidence assembled over the past 10 years substantiates that ICD interventions are frequent and highly effective in terminating potentially lethal ventricular tachyarrhythmia.16,22,24 Indeed, experience with ICDs has created a new strategy within the HCM armamentarium as the most reliable treatment currently available for SCD prevention. The most reliable data are largely confined to an international multicenter registry of 506 HCM patients with ICDs implanted for secondary and primary prevention (n = 42), on the basis of the clinical judgment of managing electrophysiologists and cardiologists (see Figure 55-2), with more than twofold the number of participants in the first Multicenter Automatic Defibrillator Implantation Trial (MADIT I).30
Over an average follow-up of 3.7 years, 20% of patients experienced appropriate device therapy for VT or ventricular fibrillation (VF), equivalent to five ICDs implanted per intervention. Appropriate ICD discharge rates were 5.5% per year, 11% per year for secondary prevention (in patients with cardiac arrest and sustained VT, respectively), and 4% per year for primary prevention (in patients with one or more risk factors) (see Figure 55-3). The highest rates are among children and adolescents (up to 11% per year), consistent with the known predilection of SCD in young patients with HCM.1–6,16,21,22,24,49 Of note, primary prevention intervention rates in high-risk patients (i.e., 4%) are similar to those previously reported for SCD from tertiary HCM centers with skewed high-risk referral patterns, and four times the SCD rates from less selective community-based patient populations.5,25,26 It should be emphasized that ICDs were effective in terminating VT or VF despite the complex HCM phenotype, which may include extreme LV hypertrophy, subaortic obstruction, microvascular ischemia, and diastolic dysfunction.1–5
Risk Period in Hypertrophic Cardiomyopathy
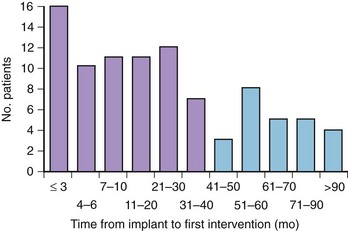
In contrast to those with ischemic heart disease, patients with HCM are exposed to an extended period of risk for SCD, which is predominant in patients younger than 30 years, but, importantly, including those in mid-life and even beyond; indeed, no particular age itself appears to confer immunity to SCD.9 Mean age at implantation is about only 40 years (almost 25% <30 years old), and the age at the time of first appropriate device intervention is also 40 years.16,22,24 An important principle related to ICDs in HCM surrounds the highly unpredictable timing of life-threatening ventricular tachyarrhythmias, which commonly have long periods of dormancy (Figure 55-4). Substantial delays of many years between implantation and initial appropriate device intervention are not uncommon (see Figure 55-4)1 and circadian patterns of ICD-terminated events do not show a discrete hourly predilection, and also occur frequently during sleep.6,22,24,50,51
Inducible Ventricular Arrhythmias
The strategy of using electrophysiological testing with programmed ventricular stimulation and inducible ventricular tachyarrhythmias to identify the substrate for and the mechanisms of SCD and to target those HCM patients at increased SCD risk has been largely abandoned.5 Limitations to this technique include the infrequency with which monomorphic VT is inducible in HCM (in only 10% of patients), and the observation that the electrical responses of the HCM substrate appear to be highly dependent on the precise stimulation protocol used.5,22,52
Complications and Other Considerations
Although ICD components have proved generally safe and effective, a number of device-related complications, including infection, pocket hematoma, pneumothorax, and venous thrombosis, do occur rarely.16,22,24,53 More frequently, about 25% of patients with HCM experience inappropriate shocks (5.3% per year) resulting from lead fracture or dislodgment, oversensing, double counting of QRS complexes, or T-wave oversensing or when triggered by sinus tachycardia or AF; multiple-shock “storms” are rare.16,22,24,53 Such complications most commonly occur in younger patients, largely because of activity level and body growth.54 Of note, young patients will require many generator changes over their lifetime, which exposes those patients to a much higher risk, on average, of lead complications and infection compared with high-risk patients with CAD.
Atrial Fibrillation
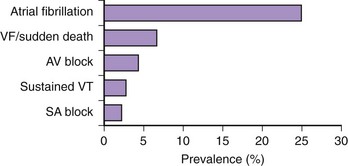
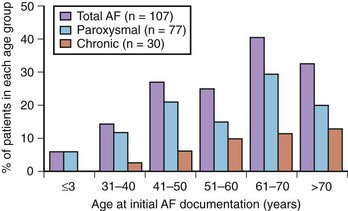
AF is the most frequent sustained arrhythmia in HCM (Figure 55-5).23 When expressed clinically, AF may have substantial prognostic importance in a considerable proportion of patients with HCM by virtue of its association with heart failure and embolic stroke in the long term and also occasionally acute hemodynamic decompensation.23,25,27,55,56 It has not, however, been conclusively determined whether AF in HCM causes heart failure or, alternatively, is a reflection of more severe disease.
Prevalence and Demographics
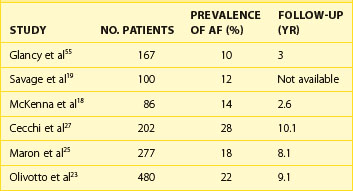
A number of centers have reported that AF occurs in HCM with a prevalence of approximately 20% to 25% and that a 2% incidence of new cases is seen annually (Table 55-1).23,27 Patients with HCM appear to have an overall four to six times greater likelihood of developing AF compared with the general population.57–61 The average age for AF onset in HCM is 55 ± 15 years, progressively increasing in frequency with age, and is predominant in those older than 60 years (see Figure 55-5). However, AF appears to be exceedingly uncommon in patients with HCM who are younger than 25 years.
Predisposing Factors
AF has some measure of predictability as a complication of HCM in that it is related to advanced age and to increased left atrial size, which is the most powerful AF predictor. Modest enlargement of the left atrium (i.e., in the range of transverse dimension 40 to 45 mm) is common, even in patients with no history of AF, probably largely as a consequence of impaired diastolic function caused by the thickened and poorly compliant ventricular chambers.23,55,62,63 However, the determinants of marked and progressive left atrial enlargement, which ultimately predispose to AF in some patients with HCM, remain unresolved (i.e., factors responsible for increased left atrial dimension >50 mm). In addition to left atrial size, atrial systolic dysfunction, which can be easily assessed by trans-thoracic echocardiography, has been shown to be a predictor of AF.64 Conversely, neither the degree of mitral regurgitation nor the presence of outflow obstruction reliably predicts the development of AF in HCM. Indeed, moderate to severe mitral regurgitation occurs in only a minority of HCM patients with AF (i.e., approximately 15%), and the proportion of patients with outflow obstruction is similar among patients with or without AF.23
Alternatively, it is possible that specific HCM-causing mutations may increase the predisposition to AF, possibly by creating an intrinsic atrial myopathy associated with prolonged and fragmented atrial conduction.65 Families commonly showing coexistence of HCM and AF have been reported to be linked to β-myosin heavy chain mutations.66 Such a hypothesis could also help explain the development of AF in the absence of left atrial enlargement, a scenario observed in about 10% of patients with HCM.23
It is not possible, on the basis of available evidence, to reliably identify those patients with HCM in sinus rhythm who will ultimately develop AF—or at least not to a degree sufficient to justify prophylactic intervention. Nevertheless, three noninvasive parameters (left atrial enlargement, P-wave prolongation on signal-averaged ECG, and supraventricular tachyarrhythmias on Holter ECG) may allow identification of a patient subset with the highest risk for developing AF in the future.67
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree